Bemotrizinol
Bemotrizinol (INN[1][2]/USAN,[3] INCI bis-ethylhexyloxyphenol methoxyphenyl triazine) is an oil-soluble organic compound that is added to sunscreens to absorb UV rays. It is marketed as Parsol Shield, Tinosorb S, and Escalol S.
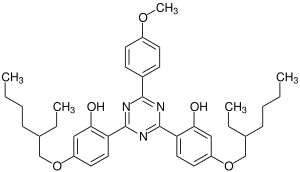 | |
| Names | |
|---|---|
| Preferred IUPAC name
2,2′-[6-(4-Methoxyphenyl)-1,3,5-triazine-2,4-diyl]bis{5-[(2-ethylhexyl)oxy]phenol} | |
| Other names
Tinosorb S Bis-ethylhexyloxyphenol methoxyphenyl triazine Anisotriazine | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
| Abbreviations | BEMT |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.109.468 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
InChI
| |
SMILES
| |
| Properties | |
Chemical formula |
C38H49N3O5 |
| Molar mass | 627.826 g·mol−1 |
| Hazards | |
| GHS labelling: | |
Hazard statements |
H413 |
Precautionary statements |
P273, P501 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Bemotrizinol is a broad-spectrum UV absorber, absorbing UVB as well as UVA rays. It has two absorption peaks, 310 and 340 nm.[4] It is highly photostable. Even after 50 MEDs (minimal erythemal doses), 98.4% remains intact. It helps prevent the photodegradation of other sunscreen actives like avobenzone.[5] A recent development is Tinosorb S Aqua, which is bemotrizinol in a PMMA matrix dispersed in water. This makes it possible to add bemotrizinol to the water phase.[6]
Bemotrizinol has strong synergistic effects on the SPF when formulated with bisoctrizole, ethylhexyl triazone or iscotrizinol.[7] It is the most effective UV absorber available measured by SPF, based on the maximum concentration permitted by European legislation.[8]
As of 2022,[9] bemotrizinol is not approved by the United States Food and Drug Administration for use in sunscreens, but has been approved in the European Union since 2000[10] and some other parts of the world, including Australia.[11][12]
Unlike some other organic sunscreen actives, it shows no estrogenic effects in vitro.[13]
References
- "Home" (PDF). Archived from the original (PDF) on 2007-09-19. Retrieved 2008-09-19.
- "Home" (PDF). Archived from the original (PDF) on 2009-10-16. Retrieved 2008-09-28.
- "Archived copy". Archived from the original on 2007-09-29. Retrieved 2007-08-19.
{{cite web}}: CS1 maint: archived copy as title (link) - Vielhaber G, Grether-Beck S, Koch O, Johncock W, Krutmann J (March 2006). "Sunscreens with an absorption maximum of > or =360 nm provide optimal protection against UVA1-induced expression of matrix metalloproteinase-1, interleukin-1, and interleukin-6 in human dermal fibroblasts". Photochem Photobiol Sci. 5 (3): 275–82. doi:10.1039/b516702g. PMID 16520862.
- Chatelain E, Gabard B (September 2001). "Photostabilization of Butyl methoxydibenzoylmethane (Avobenzone) and Ethylhexyl methoxycinnamate by Bis-ethylhexyloxyphenol methoxyphenyl triazine (Tinosorb S), a new UV broadband filter". Photochem Photobiol. 74 (3): 401–6. doi:10.1562/0031-8655(2001)074<0401:POBMAA>2.0.CO;2. PMID 11594052. S2CID 29879472.
- "basf-chemtrade.de" (PDF). www.basf-chemtrade.de. Archived from the original (PDF) on 2011-09-05. Retrieved 2011-04-15.
- "BASF – Global Home" (PDF).
- Couteau C, Pommier M, Paparis E, Coiffard LJ (June 2007). "Study of the efficacy of 18 sun filters authorized in European Union tested in vitro". Pharmazie. 62 (6): 449–52. doi:10.1691/ph.2007.6.6247. PMID 17663193.
- Mull, Amanda (1 July 2022). "You're Not Allowed to Have the Best Sunscreens in the World". The Atlantic. Retrieved 2 July 2022.
- Osterwalder, U.; Luther, H.; Herzog, B. (2001). "Über den Lichtschutzfaktor hinaus - neue effiziente und photostabile UVA-Filter". Bundesgesundheitsblatt - Gesundheitsforschung - Gesundheitsschutz. 44 (5): 463–470. doi:10.1007/s001030170019. S2CID 36985446.
- "NEW-WAVE SUNSCREENS: Active ingredient makers are frustrated by the long list of sunscreens and UV-A testing protocols that are still awaiting FDA decisions". Chemical & Engineering News. 83 (15): 18–22. April 11, 2005. doi:10.1021/cen-v083n015.p018.
- "Australian Regulatory Guidelines for OTC Medicines - Chapter 10" (PDF). Archived from the original (PDF) on August 31, 2007.
- Ashby J, Tinwell H, Plautz J, Twomey K, Lefevre PA (December 2001). "Lack of binding to isolated estrogen or androgen receptors, and inactivity in the immature rat uterotrophic assay, of the ultraviolet sunscreen filters Tinosorb M-active and Tinosorb S". Regul Toxicol Pharmacol. 34 (3): 287–91. doi:10.1006/rtph.2001.1511. PMID 11754532.