Diphyllobothrium
Diphyllobothrium is a genus of tapeworms which can cause diphyllobothriasis in humans through consumption of raw or undercooked fish. The principal species causing diphyllobothriasis is D. latum, known as the broad or fish tapeworm, or broad fish tapeworm. D. latum is a pseudophyllid cestode that infects fish and mammals. D. latum is native to Scandinavia, western Russia, and the Baltics, though it is now also present in North America, especially the Pacific Northwest. In Far East Russia, D. klebanovskii, having Pacific salmon as its second intermediate host, was identified.[1]
| Diphyllobothrium | |
|---|---|
 | |
| Proglottids of D. latum | |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Platyhelminthes |
| Class: | Cestoda |
| Order: | Diphyllobothriidea |
| Family: | Diphyllobothriidae |
| Genus: | Diphyllobothrium Cobbold, 1858 |
| Species | |
|
D. pacificum
D. ursi | |
| Synonyms | |
|
Cordicephalus Wardle, McLeod & Stewart, 1947 | |
Other members of the genus Diphyllobothrium include D. dendriticum (the salmon tapeworm), which has a much larger range (the whole northern hemisphere), D. pacificum, D. cordatum, D. ursi, D. lanceolatum, D. dalliae, and D. yonagoensis, all of which infect humans only infrequently. In Japan, the most common species in human infection is D. nihonkaiense, which was only identified as a separate species from D. latum in 1986.[2] More recently, a molecular study found D. nihonkaiense and D. klebanovskii to be a single species.[3]
Morphology

The adult worm is composed of three fairly distinct morphological segments: the scolex (head), the neck, and the lower body. Each side of the scolex has a slit-like groove, which is a bothrium for attachment to the intestine. The scolex attaches to the neck, or proliferative region. From the neck grow many proglottid segments which contain the reproductive organs of the worm. D. latum is the longest tapeworm in humans, averaging ten meters long. Unlike many other tapeworms, Diphyllobothrium eggs are typically unembryonated when passed in human feces.[4]
In adults, proglottids are wider than they are long (hence the name broad tapeworm). As in all pseudophyllid cestodes, the genital pores open midventrally.[5]
Life cycle


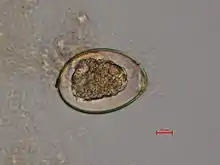
Adult tapeworms may infect humans, canids, felines, bears, pinnipeds, and mustelids, though the accuracy of the records for some of the nonhuman species is disputed. Immature eggs are passed in feces of the mammal host (the definitive host, where the worms reproduce). After ingestion by a suitable freshwater crustacean such as a copepod (the first intermediate host), the coracidia develop into procercoid larvae. Following ingestion of the copepod by a suitable second intermediate host, typically a minnow or other small freshwater fish, the procercoid larvae are released from the crustacean and migrate into the fish's flesh where they develop into a plerocercoid larvae (sparganum). The plerocercoid larvae are the infective stage for the definitive host (including humans).[6]
Because humans do not generally eat undercooked minnows and similar small freshwater fish, these do not represent an important source of infection. Nevertheless, these small second intermediate hosts can be eaten by larger predator species, for example trout, perch, walleye, and pike. In this case, the sparganum can migrate to the musculature of the larger predator fish and mammals can acquire the disease by eating these later intermediate infected host fish raw or undercooked. After ingestion of the infected fish, the plerocercoids develop into immature adults and then into mature adult tapeworms which will reside in the small intestine. The adults attach to the intestinal mucosa by means of the two bilateral grooves (bothria) of their scolices. The adults can reach more than 10 m (up to 30 ft) in length in some species such as D. latum, with more than 3,000 proglottids. One or several of the tape-like proglottid segments (hence the name tapeworm) regularly detach from the main body of the worm and release immature eggs in freshwater to start the cycle over again. Immature eggs are discharged from the proglottids (up to 1,000,000 eggs per day per worm) and are passed in the feces. The incubation period in humans, after which eggs begin to appear in the feces is typically 4–6 weeks, but can vary from as short as 2 weeks to as long as 2 years.[7]
Disease
Diphyllobothriasis is considered a parasitic, zoonotic infection. D. latum causes a wide spectrum of disease and severity. The tapeworm induces changes in the concentration of several immunomodulators in the host. It can also cause structural changes in the GI tract as it modulates neuroendocrine responses and enhances secretion and gut motility. Damage may also come from the body's immune response against the worm and its millions of eggs (around 1 million/day) mediated by mast cells, eosinophilic cell degranulations resulting to inflammatory cytokines.[8] Diphyllobothriosis is considered as the most important fish-borne zoonosis with up to 20 million individuals infected.[9]
D. latum causes B12 deficiency in humans[10] leading to megaloblastic or pernicious anemia.[11][12] The worm absorbs around 80% of dietary B12 and prolonged infection can also cause abdominal pain, mechanical obstruction, and symptoms of iron deficiency anemia.[13] Patients with prolonged infection of D. latum must also undergo B12 supplementations along with anti-parasitics such as niclosamide or praziquantel.[14]
See also
References
- Muratov, IV; Posokhov, PS (1988). "Causative agent of human diphyllobothriasis--Diphyllobothrium klebanovskii sp. n.". Parazitologiia. 22 (2): 165–70. PMID 3387122.
- Yamane, Y; Kamo, H; Bylund, G; Wikgren, BJ (1986). "Diphyllobothrium nihonkaiense sp. nov (Cestoda: Diphyllobothriidae)---revised identification of Japanese broad tapeworm". Shimane J Med Sci. 10: 29–48.
- Arizono, N; Shedko, M; Yamada, M; Uchikawa, R; Tegoshi, T; Takeda, K; Hashimoto, K (2009). "Mitochondrial DNA divergence in populations of the tapeworm Diphyllobothrium nihonkaiense and its phylogenetic relationship with Diphyllobothrium klebanovskii". Parasitology International. 58 (1): 22–8. doi:10.1016/j.parint.2008.09.001. PMID 18835460.
- Ash, Lawrence; Orihel, Thomas (2007). Ash & Orihel's Atlas of Human Parasitology (5th ed.). American Society for Clinical Pathology Press.
- Poddubnaya, Larisa G; Mackiewicz, John S; Brunanská, Magdaléna; Scholtz, Tomás (November 2005). "Fine structure of the female reproductive ducts of Cyathocephalus truncatus (Cestoda: Spathebothriidea), from salmonid fish". Folia Parasitologica. 52 (4): 323–338. doi:10.14411/fp.2005.045. PMID 16405296.
- "CDC - DPDx - Diphyllobothriasis". www.cdc.gov. 2019-05-14. Retrieved 2020-07-29.
- http://web.gideononline.com/web/epidemiology/
- Durrani MI, Basit H, Blazar E. Diphyllobothrium Latum. 2020 Jun 30. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2020 Jan–. PMID 31082015.
- Scholz T, Garcia HH, Kuchta R, Wicht B. Update on the human broad tapeworm (genus diphyllobothrium), including clinical relevance. Clin Microbiol Rev. 2009 Jan;22(1):146-60, Table of Contents. doi: 10.1128/CMR.00033-08. PMID 19136438; PMCID: PMC2620636.
- Nyberg W, Grasbeck R, Saarni M, von Bonsdorff. Serum vitamin B12 levels and incidence of tapeworm anemia in a population heavily infected with Diphyllobothrium latum. Am J Clin Nutr. 1961 Sep-Oct;9(5):606-12. doi: 10.1093/ajcn/9.5.606. PMID 13729951.
- VON BONSDORFF B. Diphyllobothrium latum as a cause of pernicious anemia. Exp Parasitol. 1956 Mar;5(2):207-30. doi: 10.1016/0014-4894(56)90015-7. PMID 13317942.
- VON BONSDORFF B, GORDIN R. Treatment of pernicious anemia with intramuscular injections of tapeworm extracts. XIV. Diphyllobothrium latum and pernicious anemia. Acta Med Scand. 1953;144(4):263-7. doi: 10.1111/j.0954-6820.1953.tb15695.x. PMID 13039956.
- Sharma K, Wijarnpreecha K, Merrell N. Diphyllobothrium latum Mimicking Subacute Appendicitis. Gastroenterology Res. 2018 Jun;11(3):235-237. doi: 10.14740/gr989w. Epub 2018 May 31. PMID 29915635; PMCID: PMC5997473.
- Vuylsteke P, Bertrand C, Verhoef GE, Vandenberghe P. Case of megaloblastic anemia caused by intestinal taeniasis. Ann Hematol. 2004 Jul;83(7):487-8. doi: 10.1007/s00277-003-0839-2. Epub 2004 Jan 17. PMID 14730392.
Sources
- "DPDx - Diphyllobothriasis". CDC Division of Parasitic Diseases. 2019-02-04. Archived from the original on 2007-11-16.
- "UDiphyllobothrium spp". Bad Bug Book. Retrieved 2009-07-13.
- Janovy, John; Roberts, Larry S. (2005). Foundations of Parasitology (7th ed.). McGraw-Hill Education (ISE Editions). ISBN 978-0-07-111271-0.
- Bonsdorff, B von: Diphyllobothriasis in Man. Academic Press, London, 1977
- Keas, B. E: Microscopy - Diphyllobothrium latum. Michigan State University, East Lancing, 1999