Parasitic worm
Parasitic worms, also known as helminths,[1] are large macroparasites; adults can generally be seen with the naked eye. Many are intestinal worms that are soil-transmitted and infect the gastrointestinal tract. Other parasitic worms such as schistosomes reside in blood vessels.
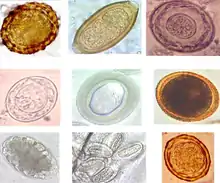
Some parasitic worms, including leeches and monogeneans, are ectoparasites – thus, they are not classified as helminths, which are endoparasites.
Parasitic worms live in and feed in living hosts. They receive nourishment and protection while disrupting their hosts' ability to absorb nutrients. This can cause weakness and disease in the host, and poses a global health and economic problem.[2] Parasitic worms cannot reproduce entirely within their host's body; they have a life cycle that includes some stages that need to take place outside of the host.[3] Helminths are able to survive in their mammalian hosts for many years due to their ability to manipulate the host's immune response by secreting immunomodulatory products.[4] All parasitic worms produce eggs during reproduction. These eggs have a strong shell that protects them against a range of environmental conditions. The eggs can therefore survive in the environment for many months or years.
Many of the worms referred to as helminths are intestinal parasites. An infection by a helminth is known as helminthiasis, helminth infection, or intestinal worm infection. There is a naming convention which applies to all helminths: the ending "-asis" (or in veterinary science: "-osis") is added at the end of the name of the worm to denote the infection with that particular worm. For example, Ascaris is the name of a type of helminth, and ascariasis is the name of the infection caused by that helminth.
Taxonomy


Helminths are a group of organisms which share a similar form but are not necessarily related as part of evolution. The term "helminth" is an artificial term.[5][6] There is no real consensus on the taxonomy (or groupings) of the helminths, particularly within the nematodes.[7] The term "helminth" contains a number of phyla, many of which are completely unrelated. However, for practical considerations the term is currently used to describe four phyla with superficial similarities: Annelida (ringed or segmented worms), Platyhelminthes (flatworms), Nematoda (roundworms), and Acanthocephala (thorny-headed worms).[7] The phylum Platyhelminthes includes two classes of worms of particular medical significance: the cestodes (tapeworms) and the trematodes (flukes and blood flukes), depending on whether or not they have segmented bodies.[1][8]
There may be as many as 300,000 species of parasites affecting vertebrates,[9] and as many as 300 affecting humans alone.[10]
Helminths of importance in the sanitation field are the human parasites, and are classified as Nemathelminthes (nematodes) and Platyhelminthes, depending on whether they possess a round or flattened body, respectively.[8]
Ringworm (dermatophytosis) is actually caused by various fungi and not by a parasitic worm.[11][12]
Reproduction and life cycle
The lifetime of adult worms varies tremendously from one species to another but is generally in the range of 1 to 8 years (see following table). This lifetime of several years is a result of their ability to manipulate the immune response of their hosts by secreting immunomodulatory products.[4]
Helminths can be either hermaphroditic (having the sex organs of both sexes), like tapeworms and flukes (not including the blood fluke), or have their sexes differentiated, like the roundworms.
All helminths produce eggs (also called ova) for reproduction.
Eggs
_(4369783299).jpg.webp)
.jpg.webp)
Generally, thousands or even hundreds of thousands of eggs are produced each time the female worm deposits its eggs - a process called oviposition. There is a large variation in the number of eggs produced by different species of worm at one time; it varies in the range of 3,000 to 700,000. The frequency of egg deposition from an adult helminth is generally daily, and can occur up to six times per day for some Taenia species. Adult trematodes lay smaller numbers of eggs compared to cestodes or nematodes. However, the egg develops into a miracidia from which thousands of cercariae, or swimming larvae, develop. This means that one egg may produce thousands of adult worms.[13] Helminth eggs remain viable for 1–2 months in crops and for many months in soil, fresh water, and sewage, or even for several years in feces, fecal sludge (historically called night soil), and sewage sludge – a period that is much longer compared to other microorganisms.[14][15]
Eggs can reach the soil when polluted wastewater, sewage sludge or human waste are used as fertilizer. Such soil is often characterized by moist and warm conditions. Therefore, the risk of using contaminated wastewater and sludge in agricultural fields is a real problem, especially in poor countries, where this practice is prevalent.[16][17] Helminth eggs are regarded as the main biological health risk when applying sewage sludge, fecal sludge or fecal matter on agricultural soils.[14] The eggs are the infective stage of the helminths’ life cycle for causing the disease helminthiasis.
Helminth eggs are resistant to various environmental conditions due to the composition of the egg shell. Each helminth egg species has 3 to 4 layers with different physical and chemical characteristics:
- the 1 to 2 outer layers are formed of mucopolysaccharides and proteins,
- the middle layers consist of chitinous material and serve to give structure and mechanical resistance to the eggs, and
- the inner layer is composed of lipids and proteins and is useful to protect eggs from desiccation, strong acid and bases, oxidants and reductive agents as well as detergent and proteolytic compounds.[16][18][19][20]
Due to this strong shell, helminth eggs or ova remain viable in soil, fresh water and sewage for many months. In feces, fecal sludge and sewage sludge they can even remain viable for several years.[14][15] Helminth eggs of concern in wastewater used for irrigation have a size between 20 and 90 μm and a relative density of 1.06–1.23.[16] It is very difficult to inactivate helminth eggs, unless temperature is increased above 40 °C or moisture is reduced to less than 5%.[16] Eggs that are no longer viable do not produce any larvae. In the case of Ascaris lumbricoides (giant roundworm), which has been considered the most resistant and common helminth type, fertilized eggs deposited in soil are resistant to desiccation but are, at this stage of development, very sensitive to environmental temperatures: The reproduction of a fertilized egg within the eggshell develops at an environmental soil temperature about 25 °C which is lower than the body temperature of the host (i.e., 37 °C for humans).[21] However, development of the larvae in the egg stops at temperatures below 15.5 °C, and eggs cannot survive temperatures much above 38 °C. If the temperature is around 25 °C, the infectiousness occurs after nearly 10 days of incubation.[8][22][23]
Larvae
Larvae hatch from eggs, either inside or outside the host, depending on the type of helminth. For eggs in moist soil at optimal temperature and oxygen levels, the embryo develops into an infective larva after 2 to 4 weeks, named "second-stage larva". Once ingested by a host, this larva has the ability to get out of the egg, hatch in the small intestine and migrate to different organs. These infective larvae (or "infective eggs") may remain viable in soil for two years or longer.[21]
The process of larval maturation in the host can take from about two weeks up to four months, depending on the helminth species.
The following table shows the principal morphological and reproductive distinctions for three helminth groups:
| Tapeworms (Cestodes) | Flukes (Trematodes) | Roundworms (Nematodes) | ||||||
|---|---|---|---|---|---|---|---|---|
| Examples | Taenia solium, Taenia saginata, Hymenolepis spp., Echinococcus granulosus, Echinococcus multilocularis, Multiceps multiceps | Schistosoma mansoni, Schistosoma japonicum,
Fasciola hepatica |
Ascaris spp., Enterobius, Filarioidea, Onchocerca spp., Rhabditis spp., Trichuris spp., Necator americanus, Ancylostoma spp. | |||||
| Pathological conditions caused in humans | Tapeworm infection, echinococcosis, alveolar echinococcosis | Schistosomiasis, swimmer's itch | Ascariasis, enterobiasis (pinworm infection, oxyuriasis), filariasis, dracunculiasis (guinea worm), elephantiasis, enterobiasis (pinworm), filariasis, hookworm infection (includes Necatoriasis and Ancylostoma duodenale infection), onchocerciasis, trichinosis, trichuriasis (whipworm) | |||||
| Shape | Segmented plane | Unsegmented plane | Cylindrical | |||||
| Body cavity | None | None | Present | |||||
| Body covering | Tegument | Tegument | Cuticle | |||||
| Digestive tube | None | Ends in cecum | Ends in anus | |||||
| Sex | Hermaphroditic | Hermaphroditic, except schistosomes which are dioecious | Dioecious | |||||
| Attachment organs | Sucker or bothridia, and rostellum with hooks | Oral sucker and ventral sucker or acetabulum | Lips, teeth, filariform extremities, and dentary plates | |||||
|
Number of species |
6000[24] | Estimated > 15,000[25] Registered > 9,000[26] | Estimated > 800,000 to 1,000,000
Registered > 25,000[25] | |||||
| Number of species known to infect humans | 40[24] | 16[25] | > 12,000[25] | |||||
| Species |
Hymenolepis nana |
Fasciola hepatica |
Hookworm |
Toxocara spp. | ||||
| Timeline of lifecycle stages | Larval formation |
Some days (eggs can survive for months)[27] |
9–15 days[24] |
18 days to several weeks[28] |
1–2 days[29] |
15–30 days[30] |
||
| Larval growth |
After hatching, the larvae develop into cysticercoid, which can survive for years in an animal[27] |
5–7 weeks as cercariae in snails and longer periods in wet environments as encysted metacercariae[13] |
10–14 days[28] |
5–10 days (after maturing can survive for weeks outside the host)[29] |
60–70 days (from hatching to mature state)[30] |
5–6 days[24] | ||
| Maturation to adult |
2 months (from cysticercoid to adult)[27] |
3–4 months[13] |
2–3 months[28] |
2–8 weeks[24] (can become dormant for months) |
||||
| Lifespan of adult worm |
4–6 weeks |
Several years[27] |
8–10 years[24] |
1–2 years[28] |
Several years[29] |
1 year[30] |
||
| Eggs laid per day | 250,000[3] to 700,000[24] | 3,000 to 25,000[25] | 3,000[3] to 250,000[24] | |||||
| Egg deposition | Frequency |
up to 6 times a day[27] |
daily[28] |
daily[29] |
daily[30] |
|||
| Number of eggs per event |
50,000-100,000[27] |
5,000-10,000[24] |
3,000-20,000[30] |
|||||
| Larvae per egg | 1 | 1 | 300 cercariae (Schistosoma), 250,000 metacercariae (Fasciola)[25] | 1 | 1 | 1 | 1 | |
Draft genomes for all categories of helminth have been sequenced in recent years and are available through the ParaSite sub-portal of WormBase.[31]
Use in medicine
Parasitic worms have been used as a medical treatment for various diseases, particularly those involving an overactive immune response.[32] As humans have evolved with parasitic worms, proponents argue they are needed for a healthy immune system.[32] Scientists are looking for a connection between the prevention and control of parasitic worms and the increase in allergies such as hay-fever in developed countries.[32] Removal of parasitic worms from areas is correlated with an increase in autoimmune disorders in those areas.[33] Parasitic worms may be able to damp down the immune system of their host, making it easier for them to live in the intestine without coming under attack.[32] This may be one mechanism for their proposed medicinal effect.
One study suggests a link between the rising rates of metabolic syndrome in the developed worlds and the largely successful efforts of Westerners to eliminate intestinal parasites. The work suggests eosinophils (a type of white blood cell) in fat tissue play an important role in preventing insulin resistance by secreting interleukin 4, which in turn switches macrophages into "alternative activation". Alternatively-activated macrophages are important to maintaining glucose homeostasis (i.e., blood sugar regulation). Helminth infection causes an increase in eosinophils. In the study, the authors fed rodents a high-fat diet to induce metabolic syndrome, and then injected them with helminths. Helminth infestation improved the rodents' metabolism.[34] The authors concluded:
Although sparse in blood of persons in developed countries, eosinophils are often elevated in individuals in rural developing countries where intestinal parasitism is prevalent and metabolic syndrome rare. We speculate that eosinophils may have evolved to optimize metabolic homeostasis during chronic infections by ubiquitous intestinal parasites….[34]
Levels of infectiousness
Helminth eggs contained in wastewater, sewage sludge or human excreta are not always infectious, i.e. able to cause the disease helminthiasis. Fertilized eggs and unfertilized eggs can exist side by side. Unfertilized eggs are identifiable under the microscope by their elongated shape. No larvae can hatch from these kinds of eggs. Therefore, unfertilized eggs do not pose a danger to human health.
Removal versus inactivation in wastewater and sludge treatment
In order to physically remove (but not inactivate) helminth eggs from wastewater, processes that remove particles, such as sedimentation, filtration or coagulation-flocculation are employed.[35][36] Therefore, waste stabilization ponds (lagoons), storage basins, constructed wetlands, rapid filtration or upflow anaerobic sludge blanket (UASB) reactors can be used.
Helminth ova cannot be inactivated with chlorine, UV light or ozone (in the latter case at least not with economical doses because >36 mg/L ozone are needed with 1 hour contact time).
Inactivation of helminth ova can be achieved in sewage sludge treatment where the temperature is increased over 40 °C or moisture is reduced to less than 5%.[16] Best results can be obtained when both of these conditions are combined for an extended period of time.[37] Details about the contact time under these conditions and other related environmental factors are generally not well-defined for every type of helminth egg species.[8] Helminth eggs are considered highly resistant biological structures.[16]
Indicator organism
Helminth eggs (or ova) are a good indicator organism to assess the safety of sanitation and reuse systems because they are the most environmentally resistant of all pathogens (viruses, bacteria, protozoa and helminths) and can in extreme cases survive for several years in soil.[14] Therefore, the presence or absence of viable helminth eggs ("viable" meaning that a larva would be able to hatch from the egg) in a sample of dried fecal matter, compost or fecal sludge is often used to assess the efficiency of diverse wastewater and sludge treatment processes in terms of pathogen removal. In particular, the number of viable Ascaris eggs is often taken as an indicator for all helminth eggs in treatment processes as they are very common in many parts of the world and relatively easy to identify under the microscope. However, the exact inactivation characteristics may vary for different types of helminth eggs.[8]
Environmental samples
For the purpose of setting treatment standards and reuse legislation, it is important to be able to determine the amount of helminth eggs in an environmental sample with some accuracy. The detection of viable helminth eggs in samples of wastewater, sludge or fresh feces (as a diagnostic tool for the infection helminthiasis) is not straight forward. In fact, many laboratories in developing countries lack the right equipment or skilled staff required to do so. An important step in the analytical methods is usually the concentration of the eggs in the sample, especially in the case of wastewater samples. A concentration step may not be required in samples of dried feces, e.g. samples collected from urine-diverting dry toilets.
Human stool samples
For medical purposes, the exact number of helminth eggs is less important and therefore most diagnoses are made simply by identifying the appearance of the worm or eggs in feces. Due to the large quantity of eggs laid, physicians can diagnose using as few as one or two fecal smears. The Kato technique (also called the Kato-Katz technique) is a laboratory method for preparing human stool samples prior to searching for parasite eggs. Eggs per gram is a laboratory test that determines the number of eggs per gram of feces in patients suspected of having a parasitological infection, such as schistosomiasis.
See also
- Helminthology – the study of parasitic worms and their effects on their hosts
References
- "CDC - Parasites - About Parasites". www.cdc.gov. 20 April 2018.
- Hildersley, Katie A.; McNeilly, Tom N.; Gillan, Victoria; Otto, Thomas D.; Löser, Stephan; Gerbe, François; Jay, Philippe; Maizels, Rick M.; Devaney, Eileen; Britton, Collette (2021). "Tuft Cells Increase Following Ovine Intestinal Parasite Infections and Define Evolutionarily Conserved and Divergent Responses". Frontiers in Immunology. 12: 781108. doi:10.3389/fimmu.2021.781108. PMC 8646091. PMID 34880874.
- "CDC Centers for Disease Control and Prevention, about parasites". CDC. Retrieved 28 November 2014.
- Jirillo, E., Magrone, T., Miragliotta, G. (2014). "Immunomodulation by Parasitic Helminths and its Therapeutic Exploitation". In: Pineda, M.A., Harnett, W. (eds.), Immune Response to Parasitic Infections (Vol. 2, pp. 175–212), Bentham eBooks, doi:10.2174/97816080598501140201, ISBN 978-1-60805-985-0.
- "Navigating the Phylogeny Wing, University of Berkeley, USA". Retrieved 19 December 2014.
- "Tree of Life web project". Retrieved 19 December 2014.
- "Schistosomiasis Research Group, University of Cambridge, UK". Archived from the original on 13 October 2014. Retrieved 19 December 2014.
- Maya C., Torner-Morales F.J., Lucario E.S., Hernández E., Jiménez B. (2012). "Viability of six species of larval and non-larval helminth eggs for different conditions of temperature, pH and dryness". Water Research. 46 (15): 4770–4782. doi:10.1016/j.watres.2012.06.014. PMID 22794801.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Dobson, A.; Lafferty, K. D.; Kuris, A. M.; Hechinger, R. F.; Jetz, W. (2008). "Homage to Linnaeus:How many parasites? How many hosts?". PNAS. 105 (Suppl 1): 11482–11489. Bibcode:2008PNAS..10511482D. doi:10.1073/pnas.0803232105. PMC 2556407. PMID 18695218.
- Cox, F. E. G. (2002). "History of Human Parasitology". Clinical Microbiology Reviews. 15 (4): 595–612. doi:10.1128/CMR.15.4.595-612.2002. PMC 126866. PMID 12364371.
- Mayo Clinic Staff. "Ringworm (body) - Symptoms and causes". Mayo Clinic. Retrieved 4 July 2018.
- "Ringworm". American Academy of Dermatology. Retrieved 4 July 2018.
- "Centers for Disease Control and Prevention: Parasites - Fascioliasis (Fasciola Infection)". Retrieved 13 January 2015.
- WHO (2006). Guidelines for the Safe Use of Wastewater, Excreta and Greywater, Volume 4 Excreta and Greywater Use in Agriculture (third ed.). Geneva: World Health Organization. ISBN 978-9241546850.
- Feachem, R., Bradley, D., Garelick, H., Mara, D. (1983). Sanitation and Disease: Health Aspects of Excreta and Wastewater Management. John Wiley and Sons, New York, NY.
- Jimenez B (2007). "Helminth ova removal from wastewater for agriculture and aquaculture reuse". Water Science & Technology. 55 (1–2): 485–493. doi:10.2166/wst.2007.046. PMID 17305174.

- Keraita B., Jiménez B., Drechsel P. (2008). Extent and Implications of Agricultural Reuse of Untreated, partly Treated and Diluted Wastewater in Developing Countries. CAB Reviews: Perspectives in Agriculture, Veterinary Science, Nutrition and Natural Resources, Vol 3, No 58, pp 1-15
- Fairweather I., Threadgold L.T. (1981). "Hymenolepis nana: the fine structure of the embryonic envelopes". Parasitology. 82 (3): 429–443. doi:10.1017/s0031182000066968. PMID 7243350.
- Lýsek H., Malínský J., Janisch R. (1985). "Ultrastructure of eggs of Ascaris lumbricoides Linnaeus, 1758. I. Egg-shells" (PDF). Folia Parasitologica. 32 (4): 381–384. PMID 4085927.
{{cite journal}}: CS1 maint: multiple names: authors list (link)
- Quilès F., Balandier J.Y., Capizzi-Banas S. (2006). "In situ characterisation of a microorganism surface by Raman microspectroscopy: the shell of Ascaris eggs". Analytical and Bioanalytical Chemistry. 386 (2): 249–255. doi:10.1007/s00216-006-0638-4. PMID 16900382. S2CID 39350893.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Bogitsh, Burton J.; Carter, Clint E.; Oeltmann, Thomas N. (2012). "General Characteristics of the Nematoda (Chapter 15), Intestinal Nematodes (Chapter 16)". Human Parasitology. UK: Academic Press. pp. 269–345. ISBN 978-0-12-415915-0.
- Alouini Z., Jemli M. (2001). "Destruction of helminth eggs by photosensitized porphyrin". Journal of Environmental Monitoring. 3 (5): 548–551. doi:10.1039/b103471p. PMID 11695127.
- Capizzi-Banas S., Deloge M., Remy M., Schwartzbrod J. (2004). "Liming as an advanced treatment for sludge sanitisation: helminth eggs elimination - Ascaris eggs as model". Water Research. 38 (14–15): 3251–3258. doi:10.1016/j.watres.2004.04.015. PMID 15276741.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Lamonthe Argumedo, R., Garcia Prieto, L. (1988). Human helminthiasis in Mexico. Treatment and prophylaxis Archived 2014-12-29 at the Wayback Machine, A.G.T. Editor, S.A., 1st edition, Mexico. (ISBN 9684630514)
- Pumarola, A., Rodríguez-Torres, A., García, R.J.A., Piedrola, A.G. (1987). Medical Microbiology and Parasitology (in Spanish), Ediciones Científicas y Técnicas, S. A., Barcelona, Spain, pp 850 - 880
- "Animal diversity web". September 2001. Retrieved 17 December 2014.
- "Centers for Disease Control and Prevention". Parasites - Taeniasis (Biology). Retrieved 22 January 2015.
- "Centers for Disease Control and Prevention: Parasites - Ascariasis". Retrieved 13 January 2015.
- "Centers for Disease Control and Prevention: Parasites - Hookworm". Retrieved 13 January 2015.
- "Centers for Disease Control and Prevention: Parasites - Trichuriasis (also known as Whipworm Infection)". Retrieved 13 January 2015.
- "WormBase ParaSite". Retrieved 15 April 2016.
- "Eat worms - feel better". BBC News. 3 December 2003. Retrieved 13 July 2011.
- Weinstock, Joel (October 2015). "Do We Need Worms to Promote Immune Health?". Clinical Reviews in Allergy & Immunology. 49 (2): 227–231. doi:10.1007/s12016-014-8458-3. PMID 25326880. S2CID 22950215. Retrieved 25 October 2020.
- Wu, Davina; et al. (8 April 2011). "Eosinophils Sustain Adipose Alternatively Activated Macrophages Associated with Glucose Homeostasis" (PDF). Science. 332 (6026): 243–247. Bibcode:2011Sci...332..243W. doi:10.1126/science.1201475. PMC 3144160. PMID 21436399. Archived from the original (PDF) on 2016-04-18. Retrieved 18 April 2011.
- Jimenez B., Chavez-Mejia A. (1997). Treatment of Mexico City Wastewater for Irrigation Purposes. Environmental Technology, Vol 18, pp 721-730
- Jiménez B., Maya C., Salgado G. (2001). The Elimination of Helminth Ova, Fecal Coliforms, Salmonella and Protozoan Cysts by Various Physicochemical Processes in Wastewater and Sludge. Water Science and Technology, Vol 43, No 12, pp 179-182 (DOI= 10.2166/wst.2001.0733)

- Schmidt, G.D., Roberts, L.S. (1981). Foundations of Parasitology, second ed. C.V. Mosby Company, 795 pp
Further reading
- Dickson Despommier, People, Parasites, and Plowshares: Learning from Our Body's Most Terrifying Invaders, Columbia University Press, 2016 (ISBN 978-0231161954).
