Leptospira interrogans
Leptospira interrogans is a species of obligate aerobic spirochaete bacteria.[1] Leptospira is one of the genera of the spirochaete phylum that causes severe mammalian infections.[2] This species is pathogenic to some wild and domestic animals, including pet dogs. It can also spread to humans through abrasions on the skin, where infection can cause flu-like symptoms with kidney and liver damage.[3] Human infections are commonly spread by contact with contaminated water or soil, often through the urine of both wild and domestic animals.[3] L. interrogans is mainly found in tropical regions, where waste treatment is underdeveloped.[3] The bacterium can live for weeks to months in ground or in water and can display many unique defense mechanisms to ensure its survival.
| Leptospira interrogans | |
|---|---|
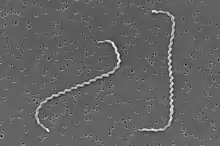 | |
| Scanning electron micrograph of Leptospira interrogans. | |
| Scientific classification | |
| Domain: | Bacteria |
| Phylum: | Spirochaetota |
| Class: | Spirochaetia |
| Order: | Leptospirales |
| Family: | Leptospiraceae |
| Genus: | Leptospira |
| Species: | L. interrogans |
| Binomial name | |
| Leptospira interrogans (Stimson 1907) Wenyon 1926 (Approved Lists 1980) | |
Biology and Biochemistry
Morphology
L. interrogans cells are gram-negative motile spirochetes, with two periplasmic flagella.[1] These flagella have polar insertions. The cells are thin, about 0.1 µm, and long, between 6-20 µm, with hooked ends. These hooked ends often resemble a question mark, and this is where the name ‘interrogans’ comes from.[4]
Metabolism/Physiology
L. interrogans displays neutralophilic properties, growing in a pH range of 7.2 - 7.6, with an optimal pH of 7.4. It also displays mesophilic growth properties, and grows at a temperature range of 28 ℃ to 30 ℃.[5][4] The optimal growth of L. interrogans occurs in simple media. Leptospira require ammonium salts as well as long-chain fatty acids for metabolism.[6]
The major energy and carbon source of this organism is the beta-oxidation of long chain fatty acids. Through naturally occurring phase interfaces or its growth media, L. interrogans must physically obtain the long chain fatty acids in order to further metabolize them as an energy source.[7] Unique to the metabolic characteristics of L. interrogans, long chain unsaturated fatty acids are required for the bacterium to grow, as saturated fatty acids can only be metabolized in these conditions.[7] L. Interrogans contains genes that code for the use of the TCA cycle in its metabolism.[7] L. interrogans' ATP production comes through oxidative phosphorylation.[7] Oxygen serves as the terminal electron acceptor in this beta-oxidation.[6] Evidence has been also shown that peroxides (ex. H2O2) can also serve as a terminal electron acceptor, with catalase activity needed for it to survive in vivo.[7] L. interrogans has only one glucose uptake system, known as the glucose sodium symporter.[8]
Genomics
The L. interrogans genome consists of two circular chromosomes and two circular chromosome composed of a total of almost 4.7 Mbp.[2] The larger chromosome has a total genome size of 4.3 Mbp, and the smaller chromosome has a size of 350-360Kbp. It has a G+C content of 35-36% and contains 3,700-4,700 protein-coding genes, depending on the strain.[8] Approximately 4,360 genes are on CI, and 367 are on CII. Only 37 of these genes encode for transfer RNAs, and the bacteria also has a low number of rRNA encoding genes. All rRNA and tRNA genes are located on CI, along with most genes related to growth. Genes specifically encoding for long-chain fatty-acid utilization, the TCA cycle, and electron transport chain have also been identified in L. interrogans. The detection of such genes confirms the use of oxidative phosphorylation as the primary metabolic pathway of L. interrogans. A large amount of genes related to eukaryotic cell invasion, cell attachment, and motility have been discovered. L. interrogans also has a complex set of genes associated with chemotaxis, more so than other pathogenic bacteria such as B. burgdorferi and T. palladium. Such genes able L. interrogans to be such a successful pathogen.[2]
Stress responses seen in L. interrogans include up-regulation expression of genes encoding proteins such as chaperone proteins including clpA, heat shock proteins including GroEL, and endoflagellar proteins including flgA.[10]
Molecular pathogenesis
Utilizing Molecular Koch's Postulates, the Loa22 gene has been classified as a virulence factor.[11]
LipL32 is a major protein in L. interrogans. Although, there is doubt regarding where this protein is located. Recent studies suggest that it is a subsurface membrane lipoprotein on the inner leaflet of the outer membrane.[12]
Some outer membrane proteins, such as OmpL1, aid in the infection process of L. interrogans by allowing adherence to host cells's surface molecules.[13]
As L. interrogans is an obligate aerobe, reactive oxygen species (ROS) must be avoided during metabolism. PerRA and PerRB are genes encoding peroxide responsive regulators, and these regulators promote host adaptation as they contain approximately 17 genes which aid in signaling.[14]
Environment
L. interrogans is a host-associated bacteria, and most infections are found in tropical regions. In the host environment, L. interrogans are first found in the blood of hosts which subsequently moves on to infect several organs. The kidney is where L. interrogans cells survive and multiply at an optimal rate. It mostly spreads through the bodily fluids of infected animals.[15] Rats are the primary carrier of leptospirosis but do not present any symptoms, transmitting the pathogen through urine, which is able to survive in freshwater.[16] The pathogen can enter the body of a new host through skin and mucous membranes, as well as through the consumption of contaminated waters.[15] Leptospira can enter the body through open cuts and other wounds, though they are unable to pass through an intact skin barrier.[16] Infected wild and domestic animals can continue to excrete the bacteria into the environment for several years, and the bacteria can survive in soil and water for months at a time.[3]
Disease
Human
In humans, symptoms caused by L. interrogans are biphasic, icteric or anicteric. The icteric form is also known as Weil’s disease.[17] Symptoms can appear anywhere between 2 to 4 weeks after exposure. Phase 1 of infection is anicteric, and symptoms include fever, chills, headache, muscle aches, vomiting and diarrhea. Roughly 90% of infectious cases in humans will only consist of this phase; however, it is possible for the disease to progress into phase 2. This is also known as the icteric phase, and the symptoms of this phase include petechiae, hepatomegaly, jaundice, renal tubular damage, hemorrhages, and subsequent renal insufficiency.[17] Drugs that have been used in the management of infection by L. interrogans include oxytetracycline, doxycycline and penicillin.[3]
There are more than 200 diverse pathogenic Leptospira serovars that make it challenging to develop an effective vaccine.[18] However, vaccines for the serovars known as Hardjo, Pomona, Canicola, Grippotyphosa and icterohaemorrhagiae have been developed. Unfortunately, these vaccines display suboptimal protection, need frequent booster doses, and are specific to certain serovars.[18]
Dog
Leptospirosis in canines can be divided into the four categories of reproductive, icteric, hemorrhagic, and uremic. Reproductive leptospirosis results in the premature birth of offspring or abortion, and uremic leptospirosis is referred to as Stuttgart disease.[19]
L. interrogans triggers a highly inflammatory response in infected dogs. This inflammatory response results in the high expression of tumor necrosis factor alpha, referred to as TNF-α, in the uterine tissue of infected dogs. Interleukin-1β and interleukein-6. also exhibit increased levels of expression upon infection. Furthermore, L. interrogans is proven to result in the down-regulation of extracellular matrix (ECM) mRNA and proteins. These factors are likely correlated with the high susceptibility of canines to leptospirosis.[19]
References
- Ren SX, Fu G, Jiang XG, Zeng R, Miao YG, Xu H, et al. (April 2003). "Unique physiological and pathogenic features of Leptospira interrogans revealed by whole-genome sequencing". Nature. 422 (6934): 888–893. Bibcode:2003Natur.422..888R. doi:10.1038/nature01597. PMID 12712204. S2CID 4415685.
- Ren, Shuang-Xi; Fu, Gang; Jiang, Xiu-Gao; Zeng, Rong; Miao, You-Gang; Xu, Hai; Zhang, Yi-Xuan; Xiong, Hui; Lu, Gang; Lu, Ling-Feng; Jiang, Hong-Quan; Jia, Jia; Tu, Yue-Feng; Jiang, Ju-Xing; Gu, Wen-Yi (April 2003). "Unique physiological and pathogenic features of Leptospira interrogans revealed by whole-genome sequencing". Nature. 422 (6934): 888–893. Bibcode:2003Natur.422..888R. doi:10.1038/nature01597. ISSN 1476-4687. PMID 12712204. S2CID 4415685.
- "Leptospirosis | CDC". www.cdc.gov. 2019-03-13. Retrieved 2021-11-23.
- Hines MT (2014). "Chapter 32 - Leptospirosis". In Sellon DC, Long MT (eds.). Equine Infectious Diseases (2nd ed.). W.B. Saunders. pp. 302–311. doi:10.1016/B978-1-4557-0891-8.00032-4. ISBN 978-1-4557-0891-8.
- Koizumi N, Watanabe H (2009). "Leptospirosis". Vaccines for Biodefense and Emerging and Neglected Diseases. Academic Press. pp. 1291–1308. doi:10.1016/B978-0-12-369408-9.00064-0. ISBN 978-0-12-369408-9.
- Evangelista KV, Coburn J (September 2010). "Leptospira as an emerging pathogen: a review of its biology, pathogenesis and host immune responses". Future Microbiology. 5 (9): 1413–1425. doi:10.2217/fmb.10.102. PMC 3037011. PMID 20860485.
- Cameron CE (2015). Alder B (ed.). "Leptospiral structure, physiology, and metabolism". Current Topics in Microbiology and Immunology. Berlin, Heidelberg: Springer. 387: 21–41. doi:10.1007/978-3-662-45059-8_3. ISBN 978-3-662-45059-8. PMID 25388131.
- Nascimento AL, Ko AI, Martins EA, Monteiro-Vitorello CB, Ho PL, Haake DA, et al. (April 2004). "Comparative genomics of two Leptospira interrogans serovars reveals novel insights into physiology and pathogenesis". Journal of Bacteriology. 186 (7): 2164–2172. doi:10.1128/JB.186.7.2164-2172.2004. PMC 374407. PMID 15028702.
- Figures, Phylogeny (2014-05-09), pntd.0002468.g001, retrieved 2022-11-02
- Lo, Miranda; Bulach, Dieter M.; Powell, David R.; Haake, David A.; Matsunaga, James; Paustian, Michael L.; Zuerner, Richard L.; Adler, Ben (2006). "Effects of Temperature on Gene Expression Patterns in Leptospira interrogans Serovar Lai as Assessed by Whole-Genome Microarrays". Infection and Immunity. 74 (10): 5848–5859. doi:10.1128/IAI.00755-06. ISSN 0019-9567. PMC 1594916. PMID 16988264.
- Verma V, Kala D, Gupta S, Kumar H, Kaushal A, Kuča K, et al. (April 2021). "Leptospira interrogans Outer Membrane Protein-Based Nanohybrid Sensor for the Diagnosis of Leptospirosis". Sensors. 21 (7): 2552. Bibcode:2021Senso..21.2552V. doi:10.3390/s21072552. PMC 8038715. PMID 33917354.
- Pinne M, Haake DA (2013-01-08). Hartskeerl RA (ed.). "LipL32 Is a Subsurface Lipoprotein of Leptospira interrogans: presentation of new data and reevaluation of previous studies". PLOS ONE. 8 (1): e51025. Bibcode:2013PLoSO...851025P. doi:10.1371/journal.pone.0051025. PMC 3544172. PMID 23323152.
- Robbins GT, Hahn BL, Evangelista KV, Padmore L, Aranda PS, Coburn J (April 2015). "Evaluation of cell binding activities of Leptospira ECM adhesins". PLOS Neglected Tropical Diseases. 9 (4): e0003712. doi:10.1371/journal.pntd.0003712. PMC 4397020. PMID 25875373.
- Grassmann AA, Zavala-Alvarado C, Bettin EB, Picardeau M, Benaroudj N, Caimano MJ (December 2021). "The FUR-like regulators PerRA and PerRB integrate a complex regulatory network that promotes mammalian host-adaptation and virulence of Leptospira interrogans". PLOS Pathogens. 17 (12): e1009078. doi:10.1371/journal.ppat.1009078. PMC 8638967. PMID 34855918.
- Johnson RC, Leptospira (1996). "Leptospira". In Baron S (ed.). Medical Microbiology (4th ed.). Galveston (TX): University of Texas Medical Branch at Galveston. ISBN 978-0-9631172-1-2. PMID 21413339.
- Eshghi A, Pappalardo E, Hester S, Thomas B, Pretre G, Picardeau M (August 2015). "Pathogenic Leptospira interrogans exoproteins are primarily involved in heterotrophic processes". Infection and Immunity. 83 (8): 3061–3073. doi:10.1128/IAI.00427-15. PMC 4496612. PMID 25987703.
- Wang S, Stobart Gallagher MA, Dunn N (2021). "Leptospirosis". StatPearls. Treasure Island (FL): StatPearls Publishing. PMID 28722888.
- Wang Z, Jin L, Wegrzyn A (December 2007). "Leptospirosis vaccines". Microbial Cell Factories. 6: 39. doi:10.1186/1475-2859-6-39. PMC 2231387. PMID 18072968.
- Wang W, Gao X, Guo M, Zhang W, Song X, Wang T, et al. (October 2014). "Leptospira interrogans induces uterine inflammatory responses and abnormal expression of extracellular matrix proteins in dogs". Microbial Pathogenesis. 75: 1–6. doi:10.1016/j.micpath.2014.07.011. PMID 25153777.