Mesenchyme
Mesenchyme (/ˈmɛsənkaɪm ˈmiːzən-/[1]) is a type of loosely organized animal embryonic connective tissue of undifferentiated cells that give rise to most tissues, such as skin, blood or bone.[2][3] The interactions between mesenchyme and epithelium help to form nearly every organ in the developing embryo.[4]
| Mesenchyme | |
|---|---|
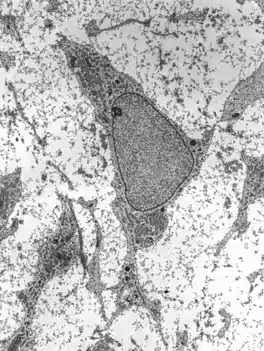 Transmission electron micrograph of mesenchyme displaying the ultrastructure of a typical cell and matrix. | |
 Mesenchyme (pointer) stained with H&E | |
| Details | |
| Carnegie stage | 6b |
| Precursor | lateral mesoderm |
| Identifiers | |
| TE | E5.16.4.0.3.0.18 |
| Anatomical terminology | |
Vertebrates
Structure
Mesenchyme is characterized morphologically by a prominent ground substance matrix containing a loose aggregate of reticular fibers and unspecialized mesenchymal stem cells.[5] Mesenchymal cells can migrate easily, in contrast to epithelial cells, which lack mobility, are organized into closely adherent sheets, and are polarized in an apical-basal orientation.
Development
The mesenchyme originates from the mesoderm.[6] From the mesoderm, the mesenchyme appears as an embryologically primitive "soup". This "soup" exists as a combination of the mesenchymal cells plus serous fluid plus the many different tissue proteins. Serous fluid is typically stocked with the many serous elements, such as sodium and chloride. The mesenchyme develops into the tissues of the lymphatic and circulatory systems, as well as the musculoskeletal system. This latter system is characterized as connective tissues throughout the body, such as bone, and cartilage. A malignant cancer of mesenchymal cells is a type of sarcoma.[7][8]
Epithelial to mesenchymal transition
The first emergence of mesenchyme occurs during gastrulation from the epithelial–mesenchymal transition (EMT) process. This transition occurs through the loss of epithelial cadherin, tight junctions, and adherens junctions on the cell membranes of epithelial cells.[9] The surface molecules undergo endocytosis and the microtubule cytoskeleton loses shape, enabling mesenchyme to migrate along the extracellular matrix (ECM). Epithelial–mesenchymal transition occurs in embryonic cells that require migration through or over tissue, and can be followed with a mesenchymal–epithelial transition to produce secondary epithelial tissues. Embryological mesenchymal cells express Protein S100-A4 (S100A4)[10] also known as fibroblast-specific protein,[11] which is indicative of their shared properties with the migratory adult fibroblasts, and c-Fos, an oncogene associated with the down-regulation of epithelial cadherin.[12][13] Both formation of the primitive streak and mesenchymal tissue is dependent on the Wnt/β-catenin pathway.[14] Specific markers of mesenchymal tissue include the additional expression of ECM factors such as fibronectin and vitronectin.[15]
Implantation
The first cells of the embryo to undergo EMT and form mesenchyme are the extra-embryonic cells of the trophectoderm. These migrate from the body of the blastocyst into the endometrial layer of the uterus in order to contribute to the formation of the anchored placenta.
Primary mesenchyme
Primary mesenchyme is the first embryonic mesenchymal tissue to emerge, and it is produced from EMT in epiblast cells. In the epiblast, it is induced by the primitive streak through Wnt signaling, and produces endoderm and mesoderm from a transitory tissue called mesendoderm during the process of gastrulation.[17]
The formation of primary mesenchyme depends on the expression of WNT3. Other deficiencies in signaling pathways, such as in Nodal (a TGF-beta protein), will lead to defective mesoderm formation.[9]
The tissue layers formed from the primitive streak invaginate together into the embryo and the induced mesenchymal stem cells will ingress and form the mesoderm. Mesodermal tissue will continue to differentiate and/or migrate throughout the embryo to ultimately form most connective tissue layers of the body.[18]
Neural mesenchyme
Embryological mesenchyme is particularly transitory and soon differentiates after migration. Neural mesenchyme forms soon after primary mesenchyme formation.[19]
The interaction with ectoderm and somite-forming morphogenic factors cause some primary mesenchyme to form neural mesenchyme, or paraxial mesoderm, and contribute to somite formation. Neural mesenchyme soon undergoes a mesenchymal–epithelial transition under the influence of WNT6 produced by ectoderm to form somites.[20] These structures will undergo a secondary EMT as the somite tissue migrates later in development to form structural connective tissue such as cartilage and skeletal muscle.[21]
Neural crest cells (NCCs) form from neuroectoderm, instead of the primary mesenchyme, from morphogenic signals of the neural crest. The EMT occurs as a result of Wnt signaling, the influence of Sox genes and the loss of E-cadherin from the cell surface. NCCs additionally require the repression of N-cadherin, and neural cell adhesion molecule. NCCs ingress into the embryo from the epithelial neuroectodermal layer and migrate throughout the body in order form multiple peripheral nervous system (PNS) cells and melanocytes. Migration of NCCs is primarily induced by BMP signaling and its inhibitor, Noggin.[22][23]
Invertebrates
In some invertebrates, e.g., Porifera, Cnidaria, Ctenophora and some triploblasts (the acoelomates), mesenchyme refers to a more-or-less solid but loosely organized tissue consisting of a gel matrix (the mesoglea) with various cellular and fibrous inclusions, located between the epidermis and the gastrodermis (non-triploblast animals usually are considered to lack "connective" tissue). In some cases, the mesoglea is noncellular.[24]
- In sponges, the mesenchyme is called mesohyl.[25]
- In diploblasts (Cnidaria and Ctenophora), the mesenchyme is fully ectodermally derived. This kind of mesenchyme is called ectomesodermal, and is not considered true mesoderm.
- In triploblastic acoelomates (such as flatworms), the term parenchyma is sometimes used for the middle (mesenchymal) layer, in which the dense layer includes tissues derived from both ectoderm, and entomesoderm (true mesoderm, derived from entoderm).
When cellular material is sparse or densely packed, as in cnidarians, the mesenchyme may sometimes be called collenchyma, or parenchyma in flatworms.[25] When no cellular material is present as in Hydrozoa), the layer is properly called mesoglea.[25]
In some colonial cnidarians, the mesenchyme is perforated by gastrovascular channels continuous among colony members. This entire matrix of common basal material is called coenenchyme.[25]
References
- "MESENCHYME English Definition and Meaning | Lexico.com". Archived from the original on September 29, 2019.
- Sadler, T. W. (2010). Langman's medical embryology (11th ed.). Philadelphia: Lippincott William & Wilkins. p. 70. ISBN 9780781790697.
- "Definition of MESENCHYME". www.merriam-webster.com.
- "Mesenchyme".
- Mesenchymal tissue
- Kierszenbaum, Abraham L.; Tres, Laura (2015). Histology and Cell Biology: An Introduction to Pathology E-Book (4 ed.). Elsevier Health Sciences. p. 123. ISBN 9780323313353.
- Strum, Judy M.; Gartner, Leslie P.; Hiatt, James L. (2007). Cell biology and histology. Hagerstown, MD: Lippincott Williams & Wilkins. p. 83. ISBN 978-0-7817-8577-8.
- Sadler, T.W. (2006). Langman's Medical Embryology. Lippincott Williams & Wilkins. pp. 68–70. ISBN 978-0-7817-9485-5.
- Kalluri, Raghu; Weinberg, Robert A. (2009). "The basics of epithelial-mesenchymal transition". Journal of Clinical Investigation. 119 (6): 1420–8. doi:10.1172/JCI39104. PMC 2689101. PMID 19487818.
- "S100A4 - Protein S100-A4 - Homo sapiens (Human) - S100A4 gene & protein". www.uniprot.org.
- Österreicher, Christoph H.; Penz-Österreicher, Melitta; Grivennikov, Sergei I. (2011-01-04). "Fibroblast-specific protein 1 identifies an inflammatory subpopulation of macrophages in the liver". Proceedings of the National Academy of Sciences. 108 (1): 308–313. Bibcode:2011PNAS..108..308O. doi:10.1073/pnas.1017547108. PMC 3017162. PMID 21173249.
- Okada, H; Danoff, T. M.; Kalluri, R; Neilson, E. G. (1997). "Early role of Fsp1 in epithelial-mesenchymal transformation". The American Journal of Physiology. 273 (4 Pt 2): F563–74. doi:10.1152/ajprenal.1997.273.4.F563. PMID 9362334.
- Eger, A; Stockinger, A; Schaffhauser, B; Beug, H; Foisner, R (2000). "Epithelial mesenchymal transition by c-Fos estrogen receptor activation involves nuclear translocation of beta-catenin and upregulation of beta-catenin/lymphoid enhancer binding factor-1 transcriptional activity". The Journal of Cell Biology. 148 (1): 173–88. doi:10.1083/jcb.148.1.173. PMC 3207144. PMID 10629227.
- Mohamed, O. A.; Clarke, H. J.; Dufort, D (2004). "Beta-catenin signaling marks the prospective site of primitive streak formation in the mouse embryo". Developmental Dynamics. 231 (2): 416–24. doi:10.1002/dvdy.20135. PMID 15366019. S2CID 39908122.
- Thiery, J. P.; Sleeman, J. P. (2006). "Complex networks orchestrate epithelial-mesenchymal transitions". Nature Reviews Molecular Cell Biology. 7 (2): 131–42. doi:10.1038/nrm1835. PMID 16493418. S2CID 8435009.
- Bellairs, R (1986). "The primitive streak". Anatomy and Embryology. 174 (1): 1–14. doi:10.1007/bf00318331. PMID 3518538. S2CID 33629601.
- Hay, E. D. (2005). "The mesenchymal cell, its role in the embryo, and the remarkable signaling mechanisms that create it". Developmental Dynamics. 233 (3): 706–20. doi:10.1002/dvdy.20345. PMID 15937929. S2CID 22368548.
- Mareschi, K; Novara, M; Rustichelli, D; Ferrero, I; Guido, D; Carbone, E; Medico, E; Madon, E; Vercelli, A; Fagioli, F (2006). "Neural differentiation of human mesenchymal stem cells: Evidence for expression of neural markers and eag K+ channel types". Experimental Hematology. 34 (11): 1563–72. doi:10.1016/j.exphem.2006.06.020. PMID 17046576.
- Schmidt, C; Stoeckelhuber, M; McKinnell, I; Putz, R; Christ, B; Patel, K (2004). "Wnt 6 regulates the epithelialisation process of the segmental plate mesoderm leading to somite formation". Developmental Biology. 271 (1): 198–209. doi:10.1016/j.ydbio.2004.03.016. PMID 15196961.
- Stockdale, F. E.; Nikovits Jr, W; Christ, B (2000). "Molecular and cellular biology of avian somite development". Developmental Dynamics. 219 (3): 304–21. doi:10.1002/1097-0177(2000)9999:9999<::AID-DVDY1057>3.0.CO;2-5. PMID 11066088. S2CID 32342256.
- Bronner-Fraser, M (1994). "Neural crest cell formation and migration in the developing embryo". FASEB Journal. 8 (10): 699–706. doi:10.1096/fasebj.8.10.8050668. PMID 8050668. S2CID 12161494.
- Trainor, P. A. (2005). "Specification of neural crest cell formation and migration in mouse embryos". Seminars in Cell & Developmental Biology. 16 (6): 683–93. doi:10.1016/j.semcdb.2005.06.007. PMID 16043371.
- Brusca, R.C.; Brusca, G.J. (2003). Invertebrates (2nd ed.). Sunderland, Massachusetts. p. 101. ISBN 9780878930975.
- Brusca, R.C.; Brusca, G.J. (2003). Invertebrates (2nd ed.). Sunderland, Massachusetts. p. 220. ISBN 9780878930975.