Tromantadine
Tromantadine is an antiviral medicine used to treat herpes simplex virus. It is available in a topical gel under trade names Viru-Merz and Viru-Merz Serol. Its performance is similar to aciclovir.[1][2]
 | |
| Clinical data | |
|---|---|
| Trade names | Viru-Merz |
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | Topical (gel) |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| NIAID ChemDB | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.053.409 |
| Chemical and physical data | |
| Formula | C16H28N2O2 |
| Molar mass | 280.412 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
Like rimantadine, amantadine, and adapromine, tromantadine is a derivative of adamantane.
Mechanism
Tromantadine inhibits the early and late events in the virus replication cycle.[3] It changes the glycoproteins of the host cells, therefore impeding the absorption of the virus. It inhibits penetration of the virus. It also prevents uncoating of the virions.
Synthesis
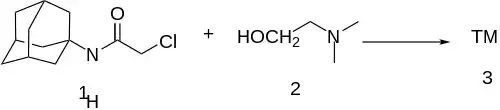
Amide formation between amantadine and chloroacetyl chloride gives N-Adamantan-1-yl-2-chloro-acetamide [5689-59-8] (1). Ether formation with Deanol (2) then completes the synthesis of tromantadine (3).
References
- Ostheimer, K. E.; Busch, T.; Görtelmeyer, R.; Hahn, K. D. (September 1989). "Randomized double-blind trial of tromantadine versus aciclovir in recurrent herpes orofacialis". Arzneimittel-Forschung. 39 (9): 1152–1155. ISSN 0004-4172. PMID 2686658.
- Diezel, W.; Michel, G.; Görtelmeyer, R.; Ostheimer, K. E. (April 1993). "Efficacy of tromantadine and aciclovir in the topical treatment of recurrent herpes orofacialis. Comparison in a clinical trial". Arzneimittel-Forschung. 43 (4): 491–496. ISSN 0004-4172. PMID 8494582.
- Rosenthal KS, Sokol MS, Ingram RL, Subramanian R, Fort RC (December 1982). "Tromantadine: inhibitor of early and late events in herpes simplex virus replication". Antimicrob. Agents Chemother. 22 (6): 1031–6. doi:10.1128/aac.22.6.1031. PMC 185716. PMID 6297383.
- Peteri D, Sterner W. [Chemistry and toxicology of a new antiviral agent: N-(1-adamantyl)-2-(2-dimethylamino-ethoxy)-acetamide-HCl.1]. Arzneimittelforschung. 1973;23(4):577-81. PMID: 4740238.
- May G, Peteri D. [Synthesis and antiviral effects of adamantan derivatives]. Arzneimittelforschung. 1973;23(5):718-21. PMID: 4351043.
- DE1941218 idem Dezso Peteri, Arthur Scherm, U.S. Patent 3,705,194 (1971 to Merz & Co).
- U.S. Patent 20,140,045,779 idem Lifeng Xu, 徐利锋, WO 2012145981 (2014 to Liaoning Lifeng Scientific & Technology Development Company Ltd., 1 More ») Ex 18.