Zona pellucida
The zona pellucida (plural zonae pellucidae, also egg coat or pellucid zone) is a specialized extracellular matrix that surrounds the plasma membrane of mammalian oocytes. It is a vital constitutive part of the oocyte. The zona pellucida first appears in unilaminar primary oocytes. It is secreted by both the oocyte and the ovarian follicles. The zona pellucida is surrounded by the corona radiata. The corona is composed of cells that care for the egg when it is emitted from the ovary.[1]
| Zona pellucida | |
|---|---|
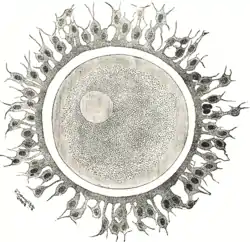 Human ovum: The zona pellucida is seen as a thick clear girdle surrounded by the cells of the corona radiata. | |
| Identifiers | |
| MeSH | D015044 |
| FMA | 18674 |
| Anatomical terminology | |
This structure binds spermatozoa, and is required to initiate the acrosome reaction. In the mouse (the best characterised mammalian system), the zona glycoprotein, ZP3, is responsible for sperm binding, adhering to proteins on the sperm plasma membrane. ZP3 is then involved in the induction of the acrosome reaction, whereby a spermatozoon releases the contents of the acrosomal vesicle. The exact characterisation of what occurs in other species has become more complicated as further zona proteins have been identified.[2][3]
In humans, five days after the fertilization, the blastocyst performs zona hatching; the zona pellucida degenerates and decomposes, to be replaced by the underlying layer of trophoblastic cells. The zona pellucida is essential for oocyte growth and fertilization.
Structure
The zona pellucida is a translucent matrix of cross-linked glycoprotein filaments that surrounds the mammalian oocyte and is 6.5–20 μm thick depending on the species. Its formation, which depends on a conserved Zona pellucida-like (ZP) module that mediates the polymerization of egg coat components,[4] is critical to successful fertilization.[5] In non-mammals it is called the vitelline membrane or vitelline envelope.[6]
Function
The thick membrane of the zona pellucida functions to only allow species-specific fertilization; to prevent polyspermy, and enable the acrosome reaction for the successful adhesion and penetration by the sperm cell. Also allows correct embryo development and size. The major glycoproteins of the egg coat responsible, are known as sperm-binding proteins.[7]
The four major sperm-binding proteins, or sperm-receptors, are ZP1, ZP2, ZP3, and ZP4. They bind to capacitated spermatozoa and induce the acrosome reaction. Successful fertilization depends on the ability of sperm to penetrate the extracellular matrix of the zona pellucida that surrounds the egg. In the mouse:
- ZP3 allows species-specific sperm binding
- ZP2 mediates subsequent sperm binding
- ZP1 cross-links ZP2 and ZP3.
Data with native human protein are not currently available.
Immunocontraception
ZP module-containing glycoproteins ZP1, ZP2, ZP3 and ZP4 are targets for immunocontraception in mammals.
In non-mammals, the zona pellucida is called the vitelline membrane or envelope, and the vitelline envelope in insects, and plays an important role in preventing cross-breeding of different species, especially in species such as fish that fertilize outside of the body.
The zona pellucida is commonly used to control wildlife population problems by immunocontraception. When the zona pellucida of one animal species is injected into the bloodstream of another, it results in sterility of the second species due to immune response. This effect can be temporary or permanent, depending on the method used. In New Jersey, immunocontraception using porcine zona pellucida has been trialled for the control of deer.[8]
Additional images
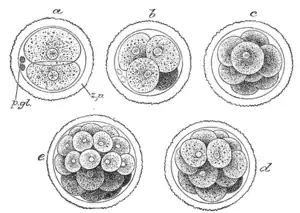 First stages of segmentation of a mammalian ovum
First stages of segmentation of a mammalian ovum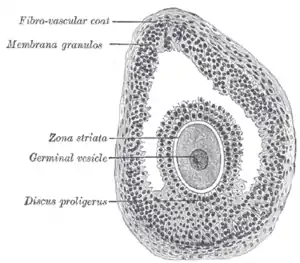 Section of vesicular ovarian follicle of cat, x 50
Section of vesicular ovarian follicle of cat, x 50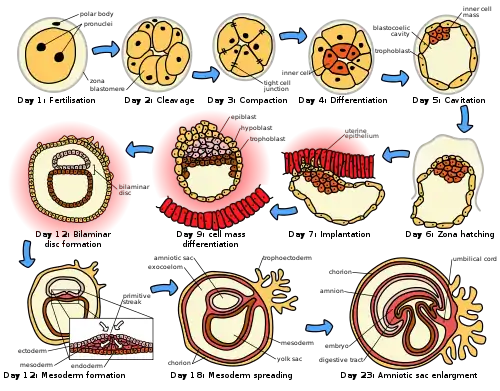 The initial stages of human embryogenesis
The initial stages of human embryogenesis
References
- Gilbert, Scott (2013). Developmental Biology. Sinauer Associates Inc. p. 123. ISBN 9781605351926.
- Conner, SJ; Hughes, DC (2003). "Analysis of fish ZP1/ZPB homologous genes--evidence for both genome duplication and species-specific amplification models of evolution". Reproduction. 126 (3): 347–52. doi:10.1530/rep.0.1260347. PMID 12968942.
- Conner, S.J.; Lefièvre, L; Hughes, DC; Barratt, CL (2005). "Cracking the egg: Increased complexity in the zona pellucida". Human Reproduction. 20 (5): 1148–52. doi:10.1093/humrep/deh835. PMID 15760956.
- Jovine L, Qi H, Williams Z, Litscher E, de Sanctis D, Wassarman PM (2002). "The ZP domain is a conserved module for polymerization of extracellular proteins". Nat. Cell Biol. 4 (6): 457–461. doi:10.1038/ncb802. PMID 12021773.
- Gupta, SK; et al. (September 2012). "Mammalian zona pellucida glycoproteins: structure and function during fertilization". Cell and Tissue Research. 349 (3): 665–78. doi:10.1007/s00441-011-1319-y. PMID 22298023. S2CID 16174953.
- Monné, M; Jovine, L (October 2011). "A structural view of egg coat architecture and function in fertilization". Biology of Reproduction. 85 (4): 661–9. doi:10.1095/biolreprod.111.092098. PMID 21715714.
- Gupta, SK; Bansal, P; Ganguly, A; Bhandari, B; Chakrabarti, K (December 2009). "Human zona pellucida glycoproteins: functional relevance during fertilization". Journal of Reproductive Immunology. 83 (1–2): 50–5. doi:10.1016/j.jri.2009.07.008. PMID 19850354.
- "Community-Based Deer Management". New Jersey Department of Environmental Protection. 24 September 2014. Retrieved 8 July 2015.
Further reading
- Bork, Peer; Sander, Chris (1992). "A large domain common to sperm receptors (Zp2 and Zp3) and TGF-β type III receptor". FEBS Letters. 300 (3): 237–40. doi:10.1016/0014-5793(92)80853-9. PMID 1313375.
- Oehninger, Sergio (2003). "Biochemical and functional characterization of the human zona pellucida". Reproductive BioMedicine Online. 7 (6): 641–8. doi:10.1016/S1472-6483(10)62086-X. PMID 14748962.
- Boja, E. S.; Hoodbhoy, T; Fales, HM; Dean, J (2003). "Structural Characterization of Native Mouse Zona Pellucida Proteins Using Mass Spectrometry". Journal of Biological Chemistry. 278 (36): 34189–202. doi:10.1074/jbc.M304026200. PMID 12799386.
- Bagnell C (2005). "Animal Reproduction". Rutgers University Department of Animal Sciences.
- Jovine, Luca; Darie, Costel C.; Litscher, Eveline S.; Wassarman, Paul M. (2005). "Zona Pellucida Domain Proteins". Annual Review of Biochemistry. 74: 83–114. doi:10.1146/annurev.biochem.74.082803.133039. PMID 15952882.
- Monné, Magnus; Han, Ling; Jovine, Luca (2006). "Tracking Down the ZP Domain: From the Mammalian Zona Pellucida to the Molluscan Vitelline Envelope". Seminars in Reproductive Medicine. 24 (4): 204–16. doi:10.1055/s-2006-948550. hdl:11563/8929. PMID 16944418.
- Wassarman, P. M. (2008). "Zona Pellucida Glycoproteins". Journal of Biological Chemistry. 283 (36): 24285–9. doi:10.1074/jbc.R800027200. PMC 2528931. PMID 18539589.
- Wassarman, Paul M.; Litscher, Eveline S. (2008). "Mammalian fertilization:the eggs multifunctional zona pellucida". The International Journal of Developmental Biology. 52 (5–6): 665–76. doi:10.1387/ijdb.072524pw. PMID 18649280.
- Monné, Magnus; Han, Ling; Schwend, Thomas; Burendahl, Sofia; Jovine, Luca (2008). "Crystal structure of the ZP-N domain of ZP3 reveals the core fold of animal egg coats". Nature. 456 (7222): 653–7. Bibcode:2008Natur.456..653M. doi:10.1038/nature07599. hdl:11563/8930. PMID 19052627. S2CID 4430083.
- Han, Ling; Monné, Magnus; Okumura, Hiroki; Schwend, Thomas; Cherry, Amy L.; Flot, David; Matsuda, Tsukasa; Jovine, Luca (2010). "Insights into Egg Coat Assembly and Egg-Sperm Interaction from the X-Ray Structure of Full-Length ZP3". Cell. 143 (3): 404–15. doi:10.1016/j.cell.2010.09.041. PMID 20970175. S2CID 18583237.
External links
- Histology image: 18404loa – Histology Learning System at Boston University - "Female Reproductive System: ovary, cumulus oophorus "
- Histology image: 14805loa – Histology Learning System at Boston University - "Female Reproductive System: ovary, multilaminar primary follicle"
- Anatomy photo: Reproductive/mammal/ovary2/ovary7 - Comparative Organology at University of California, Davis - "Mammal, canine ovary (LM, High)"
- Image at um.edu.mt
- Image at um.edu.mt