1,4-Butanediol
1,4-Butanediol, colloquially known as BD or BDO, is a primary alcohol, and an organic compound, with the formula HOCH2CH2CH2CH2OH. It is a colorless viscous liquid. It is one of four stable isomers of butanediol.
 | |
| Names | |
|---|---|
| Preferred IUPAC name
Butane-1,4-diol | |
| Other names
Tetramethylene glycol | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.003.443 |
| EC Number |
|
PubChem CID |
|
| RTECS number |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties[1][2] | |
| C4H10O2 | |
| Molar mass | 90.122 g·mol−1 |
| Density | 1.0171 g/cm3 (20 °C) |
| Melting point | 20.1 °C (68.2 °F; 293.2 K) |
| Boiling point | 235 °C (455 °F; 508 K) |
| Miscible | |
| Solubility in ethanol | Soluble |
| -61.5·10−6 cm3/mol | |
Refractive index (nD) |
1.4460 (20 °C) |
| Hazards[3][4] | |
| GHS labelling: | |
 | |
| Warning | |
Hazard statements |
H302, H336 |
Precautionary statements |
P261, P264, P270, P271, P301+P312, P304+P340, P312, P330, P403+P233, P405, P501 |
| NFPA 704 (fire diamond) | |
| Flash point | (open cup) 121 °C (250 °F; 394 K) |
Autoignition temperature |
350 °C (662 °F; 623 K) |
| Related compounds | |
Related butanediols |
1,2-Butanediol 1,3-Butanediol 2,3-Butanediol cis-Butene-1,4-diol |
Related compounds |
Succinaldehyde Succinic acid |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Synthesis
In industrial synthesis, acetylene reacts with two equivalents of formaldehyde to form 1,4-butynediol. Hydrogenation of 1,4-butynediol gives 1,4-butanediol. It is also manufactured on an industrial scale from maleic anhydride in the Davy process, which is first converted to the methyl maleate ester, then hydrogenated. Other routes are from butadiene, allyl acetate and succinic acid.
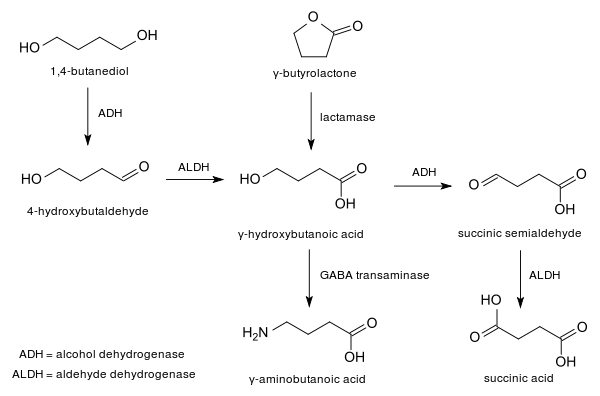
A biological route to BD has been commercialized that uses a genetically modified organism.[5] The biosynthesis proceeds via 4-hydroxybutyrate.
Industrial use
1,4-Butanediol is used industrially as a solvent and in the manufacture of some types of plastics, elastic fibers and polyurethanes. In organic chemistry, 1,4-butanediol is used for the synthesis of γ-butyrolactone (GBL). In the presence of phosphoric acid and high temperature, it dehydrates to the important solvent tetrahydrofuran.[6] At about 200 °C in the presence of soluble ruthenium catalysts, the diol undergoes dehydrogenation to form butyrolactone.[7] It is used to synthesize 1,4-Butanediol diglycidyl ether which is then used as a reactive diluent for epoxy resins.[8]
World production of 1,4-butanediol was claimed to be about one million metric tons per year and market price is about US$2,000 (€1,600) per ton (2005). In 2013, worldwide production was claimed to be billions of lbs (consistent with approximately one million metric tons).[9]
Almost half of it is dehydrated to tetrahydrofuran to make fibers such as Spandex.[10] The largest producer is BASF.[11]
Use as a recreational drug

1,4-Butanediol is also used as a recreational drug known by some users as "One Comma Four", "Liquid Fantasy", "One Four Bee" or "One Four B-D-O". A few Federal Courts have stated that 1,4-butanediol exerts effects similar to γ-hydroxybutyrate (GHB), which is a metabolic product of 1,4-butanediol. But other Federal Courts have ruled that it is not.
Pharmacokinetics
1,4-Butanediol is rapidly converted into GHB by the enzymes alcohol dehydrogenase and aldehyde dehydrogenase, and differing levels of these enzymes may account for differences in effects and side effects between users.[12] While co-administration of ethanol and GHB already poses serious risks, co-administration of ethanol with 1,4-butanediol will interact considerably and has many other potential risks. This is because the same enzymes that are responsible for metabolizing alcohol also metabolize 1,4-butanediol so there is a strong chance of a dangerous drug interaction.[12][13] Emergency room patients who overdose on both ethanol and 1,4-butanediol often present with symptoms of alcohol intoxication initially and as the ethanol is metabolized the 1,4-butanediol is then able to better compete for the enzyme and a second period of intoxication ensues as the 1,4-butanediol is converted into GHB.[12]
Pharmacodynamics
1,4-Butanediol seems to have two types of pharmacological actions. The major psychoactive effects of 1,4-butanediol are because it is metabolized into GHB; however there is a study suggesting that 1,4-butanediol may have potential alcohol-like pharmacological effects on its own.[13] The study arrived at this conclusion based on the finding that 1,4-butanediol co-administered with ethanol led to potentiation of some of the behavioral effects of ethanol. However, potentiation of ethanol's effects may simply be caused by competition for the alcohol dehydrogenase and aldehyde dehydrogenase enzymes with co-administered 1,4-butanediol. The shared metabolic rate-limiting steps thus leads to slowed metabolism and clearance for both compounds including ethanol's known toxic metabolite acetaldehyde.
Another study found no effect following intracerebroventricular injection in rats of 1,4-butanediol.[14] This contradicts the hypothesis of 1,4-butanediol having inherent alcohol-like pharmacological effects.
Like GHB, 1,4-butanediol is only safe in small amounts. Adverse effects in higher doses include nausea, vomiting, dizziness, sedation, vertigo, and potentially death if ingested in large amounts. Anxiolytic effects are diminished and side effects increased when used in combination with alcohol.
Legality
While 1,4-butanediol is not currently scheduled federally in the United States,[15] a number of states have classified 1,4-butanediol as a controlled substance. Individuals have been prosecuted for possession of 1,4-butanediol under the Federal Analog Act as substantially similar to GHB.[16] A federal case in New York in 2002 ruled that 1,4-butanediol could not be considered an analog of GHB under federal law,[17] but that decision was later overturned by the Second Circuit.[18] However, a jury in Federal District Court in Chicago found that 1,4-butanediol was not be an analog of GHB under federal law, and the Seventh Circuit Court of Appeals upheld that verdict.[19] ! In the United Kingdom, 1,4-butanediol was scheduled in December 2009 (along with another GHB precursor, gamma-butyrolactone) as a Class C controlled substance. In Germany, the drug is not explicitly illegal, but might also be treated as illegal if used as a drug. It is controlled as a Schedule VI precursor in Canada.
2007 contamination of Bindeez toy
A toy called "Bindeez" ("Aqua Dots" in North America) was recalled by the distributor in November 2007 because of the presence of 1,4-butanediol. The toy consists of small beads that stick to each other by sprinkling water. 1,4-Butanediol was detected by GC-MS.[20] The production plant seems to have intended to cut costs by replacing less toxic 1,5-pentanediol with 1,4-butanediol. ChemNet China listed the price of 1,4-butanediol at between about US$1,350–2,800 per metric ton, while the price for 1,5-pentanediol is about US$9,700 per metric ton.[21]
2021 poisoning at Darmstadt Technical University
In August 2021, several people fell severely ill after consuming drinks at building L2.01 at the Lichtwiese Campus of Darmstadt Technical University, Germany. Seven showed severe symptoms, two were transported to a hospital in Frankfurt, and a 30-year-old person was, for a time, in a critical state. 1,4-butanediol had been detected in milk packages, as well as in water filters. At the location, detectives also found bromophenols and dicyclohexylamine.[22]
See also
- 1,4-Butanediol diglycidyl ether
References
- Weast, Robert C., ed. (1981). CRC Handbook of Chemistry and Physics (62nd ed.). Boca Raton, FL: CRC Press. p. C-190. ISBN 0-8493-0462-8..
- 1,4-Butanediol, International Chemical Safety Card 1104, Geneva: International Programme on Chemical Safety, March 1999
- HSNO Chemical Classification Information Database, New Zealand Environmental Protection Authority
- "1,4-Butanediol Laboratory Chemical Safety Summary".
- "United States Patent: 8067214 - Compositions and methods for the biosynthesis of 1,4-butanediol and its precursors". uspto.gov. Retrieved 1 April 2018.
- Karas, L.; Piel, W. J., "Ethers", Kirk-Othmer Encyclopedia of Chemical Technology, New York: John Wiley, doi:10.1002/0471238961, ISBN 9780471238966
- Zhao, Jing; Hartwig, John F. (2005), "Acceptorless, neat, ruthenium-catalyzed dehydrogenative cyclization of diols to lactones", Organometallics, 24 (10): 2441–46, doi:10.1021/om048983m
- Monte, Salvatore J. (1998), Pritchard, Geoffrey (ed.), "Diluents and viscosity modifiers for epoxy resins", Plastics Additives: An A-Z reference, Polymer Science and Technology Series, Dordrecht: Springer Netherlands, vol. 1, pp. 211–216, doi:10.1007/978-94-011-5862-6_24, ISBN 978-94-011-5862-6, retrieved 2022-03-29
- "Commercial-scale production of bio-based BDO announced", Chemical Engineering, Feb 2013, archived from the original on 2015-02-04, retrieved 2013-02-21
- "Butanediol (price and demand in market)", Chemical Week, 12 April 2006, retrieved 2008-11-21
- "Malaysia: New 1,4-butanediol plant used below capacity", Asian Textile Business, April 2004, retrieved 2008-11-21
- Benzer, Theodore I.; Cameron, Scott; Russi, Christopher Scott (8 January 2007), Toxicity, Gamma-Hydroxybutyrate, eMedicine, retrieved 2009-08-29
- Poldrugo, Flavio; Snead III, O. Carter (1984), "1,4-butanediol, γ-hydroxybutyric acid and ethanol: Relationships and interactions", Neuropharmacology, 23 (1): 109–13, doi:10.1016/0028-3908(84)90226-0, PMID 6717752, S2CID 54415695
- Carter, LP; Koek, W; France, CP (2006), "Lack of effects of GHB precursors GBL and 1,4-BD following i.c.v. Administration in rats", The European Journal of Neuroscience, 24 (9): 2595–600, doi:10.1111/j.1460-9568.2006.05146.x, PMID 17100847, S2CID 24609982
- "21 U.S. Code § 841 - Prohibited acts A". LII / Legal Information Institute. Retrieved 2016-08-02.
- USA v Washam (2002) 312 F.3d 926, 930; http://cases.justia.com/us-court-of-appeals/F3/312/926/608696/
- "Erowid 1,4-Butanediol Vault : Law : New York Federal Court Rules Analogue Act Unconstitutionally Vague with regard to 1,4-Butanediol". www.erowid.org. Retrieved 1 April 2018.
- United States v. Roberts, 363 F.3d 118 (2d Cir. 2004); https://scholar.google.com/scholar_case?case=13457043198797192346&q=363+F.3d+118&hl=en&as_sdt=6,39
- United States v. Turcotte, 405 F.3d 515 (7th Cir. 2005) "With specific regard to 1,4 Butanediol, the jury has returned a special verdict which states that 1,4-Butanediol is not a Schedule I Narcotic Drug Controlled Substance analogue, because 1,4-Butanediol's chemical structure is not significantly similar to the chemical structure of GHB.
- Wang, Linda (9 November 2007), "Industrial Chemical Sullies Popular Children's Toy", Chemical & Engineering News, retrieved 2009-08-11
- "US mother says her son began to stumble and vomit after eating Chinese-made toy, now recalled", Boston Herald, Associated Press, 8 November 2007
- "Ermittler finden nach Vergiftungen an TU Darmstadt offenbar K.-o.-Tropfen in Küche". Stern. 2021-08-27. Retrieved 2021-09-01.
External links
- International Chemical Safety Card 1104
- SIDS Initial Assessment Report for 1,4-Butanediol from the Organisation for Economic Co-operation and Development (OECD)
- Record in the Household Products Database of NLM
