Ammonium nitrate
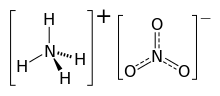
Ammonium nitrate is a chemical compound with the chemical formula NH4NO3. It is a white crystalline salt consisting of ions of ammonium and nitrate. It is highly soluble in water and hygroscopic as a solid, although it does not form hydrates. It is predominantly used in agriculture as a high-nitrogen fertilizer.[4] Global production was estimated at 21.6 million tonnes in 2017.[5]
 | |
 | |
 | |
| Names | |
|---|---|
| IUPAC name
Ammonium nitrate | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.026.680 |
| EC Number |
|
PubChem CID |
|
| RTECS number |
|
| UNII | |
| UN number | 0222 – with > 0.2% combustible substances 1942 – with ≤ 0.2% combustible substances 2067 – fertilizers 2426 – liquid |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| NH4NO3 | |
| Molar mass | 80.043 g/mol |
| Appearance | white crystalline solid |
| Density | 1.725 g/cm3 (20 °C) |
| Melting point | 169.6 °C (337.3 °F; 442.8 K) |
| Boiling point | approx. 210 °C (410 °F; 483 K) decomposes |
| Endothermic 118 g/100 ml (0 °C) 150 g/100 ml (20 °C) 297 g/100 ml (40 °C) 410 g/100 ml (60 °C) 576 g/100 ml (80 °C) 1024 g/100 ml (100 °C)[1] | |
| -33.6·10−6 cm3/mol | |
| Structure | |
| trigonal | |
| Explosive data | |
| Shock sensitivity | very low |
| Friction sensitivity | very low |
| Detonation velocity | 2500 m/s |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards |
Explosive, Oxidizer |
| GHS labelling: | |
   | |
| Danger | |
Hazard statements |
H201, H271, H319 |
Precautionary statements |
P220, P221, P264, P271, P280, P372 |
| NFPA 704 (fire diamond) | |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose) |
2085–5300 mg/kg (oral in rats, mice)[2] |
| Related compounds | |
Other anions |
Ammonium nitrite |
Other cations |
Sodium nitrate Potassium nitrate Hydroxylammonium nitrate |
Related compounds |
Ammonium perchlorate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Its other major use is as a component of explosive mixtures used in mining, quarrying, and civil construction. It is the major constituent of ANFO, a popular industrial explosive which accounts for 80% of explosives used in North America; similar formulations have been used in improvised explosive devices.
Many countries are phasing out its use in consumer applications due to concerns over its potential for misuse.[6] Accidental ammonium nitrate explosions have killed thousands of people since the early 20th century.[6]
Occurrence
Ammonium nitrate is found as the natural mineral gwihabaite (formerly known as nitrammite)[7] – the ammonium analogue of saltpetre (mineralogical name: niter)[8][9] – in the driest regions of the Atacama Desert in Chile, often as a crust on the ground or in conjunction with other nitrate, iodate, and halide minerals. Ammonium nitrate was mined there until the Haber–Bosch process made it possible to synthesize nitrates from atmospheric nitrogen, thus rendering nitrate mining obsolete.
Production, reactions and crystalline phases
The industrial production of ammonium nitrate entails the acid-base reaction of ammonia with nitric acid:[10]
- HNO3 + NH3 → NH4NO3
Ammonia is used in its anhydrous form (a gas) and the nitric acid is concentrated. The reaction is violent owing to its highly exothermic nature. After the solution is formed, typically at about 83% concentration, the excess water is evaporated off to leave an ammonium nitrate (AN) content of 95% to 99.9% concentration (AN melt), depending on grade. The AN melt is then made into "prills" or small beads in a spray tower, or into granules by spraying and tumbling in a rotating drum. The prills or granules may be further dried, cooled, and then coated to prevent caking. These prills or granules are the typical AN products in commerce.
The ammonia required for this process is obtained by the Haber process from nitrogen and hydrogen. Ammonia produced by the Haber process can be oxidized to nitric acid by the Ostwald process. Another production method is a variant of the nitrophosphate process:
The products, calcium carbonate and ammonium nitrate, may be separately purified or sold combined as calcium ammonium nitrate.
Ammonium nitrate can also be made via metathesis reactions:
Reactions
As ammonium nitrate is a salt, both the cation, NH4+, and the anion, NO3−, may take part in chemical reactions.
Solid ammonium nitrate decomposes on heating. At temperatures below around 300 °C, the decomposition mainly produces nitrous oxide and water:
- NH4NO3 → N2O + 2H2O
At higher temperatures, the following reaction predominates.[11]
- 2NH4NO3 → 2N2 + O2 + 4H2O
Both decomposition reactions are exothermic and their products are gas. Under certain conditions, this can lead to a runaway reaction, with the decomposition process becoming explosive.[12] See § Disasters for details. Many ammonium nitrate disasters, with loss of lives, have occurred.
The red–orange colour in an explosion cloud is due to nitrogen dioxide, a secondary reaction product.[12]
Crystalline phases
A number of crystalline phases of ammonium nitrate have been observed. The following occur under atmospheric pressure.
Phase Temperature (°C) Symmetry (liquid) (above 169.6) I 169.6 to 125.2 cubic II 125.2 to 84.2 tetragonal III 84.2 to 32.3 α-rhombic IV 32.3 to −16.8 β-rhombic V below −16.8 tetragonal[13]
The transition between β-rhombic to α-rhombic forms (at 32.3°C) occurs at ambient temperature in many parts of the world. These forms have a 3.6% difference in density and hence transition between them causes a change in volume. One practical consequence of this is that ammonium nitrate cannot be used as a solid rocket motor propellant, as it develops cracks. Stabilized ammonium nitrate (PSAN) was developed as a solution to this and incorporates metal halides stabilisers, which prevent density fluctuations.[14]
Applications
Fertilizer
Ammonium nitrate is an important fertilizer with NPK rating 34-0-0 (34% nitrogen).[15] It is less concentrated than urea (46-0-0), giving ammonium nitrate a slight transportation disadvantage. Ammonium nitrate's advantage over urea is that it is more stable and does not rapidly lose nitrogen to the atmosphere.
Explosives
Ammonium nitrate readily forms explosive mixtures with varying properties when combined with explosives such as TNT or with fuels like aluminum powder or fuel oil. Examples of explosives containing ammonium nitrate include:
- Astrolite (ammonium nitrate and hydrazine rocket fuel)
- Amatol (ammonium nitrate and TNT)
- Ammonal (ammonium nitrate and aluminum powder)
- Amatex (ammonium nitrate, TNT and RDX)
- ANFO (ammonium nitrate and fuel oil)
- DBX (ammonium nitrate, RDX, TNT and aluminum powder)
- Tovex (ammonium nitrate and methylammonium nitrate)
- Minol (explosive) (ammonium nitrate, TNT and aluminum powder)
- Goma-2 (ammonium nitrate, nitroglycol, Nitrocellulose, Dibutyl phthalate and fuel)
Mixture with fuel oil
ANFO is a mixture of 94% ammonium nitrate ("AN") and 6% fuel oil ("FO") widely used as a bulk industrial explosive.[16]: 1 It is used in coal mining, quarrying, metal mining, and civil construction in undemanding applications where the advantages of ANFO's low cost, relative safety, and ease of use matter more than the benefits offered by conventional industrial explosives, such as water resistance, oxygen balance, high detonation velocity, and performance in small diameters.[16]: 2
Terrorism
Ammonium nitrate-based explosives were used in the Sterling Hall bombing in Madison, Wisconsin, 1970, the Oklahoma City bombing in 1995, the 2011 Delhi bombings, the 2011 bombing in Oslo, and the 2013 Hyderabad blasts.
In November 2009, the government of the North West Frontier Province (NWFP) of Pakistan imposed a ban on ammonium sulfate, ammonium nitrate, and calcium ammonium nitrate fertilizers in the former Malakand Division – comprising the Upper Dir, Lower Dir, Swat, Chitral, and Malakand districts of the NWFP – following reports that those chemicals were used by militants to make explosives. Due to these bans, "Potassium chlorate – the stuff that makes safety matches catch fire – has surpassed fertilizer as the explosive of choice for insurgents."[17]
Niche uses
Ammonium nitrate is used in some instant cold packs, as its dissolution in water is highly endothermic. In 2021, King Abdullah University of Science and Technology in Saudi Arabia conducted experiments to study the potential for dissolving ammonium nitrate in water for off-grid cooling systems and as a refrigerant. They suggested that the water could be distilled and reused using solar energy to avoid water wastage in severe environments.[18]
It was once used, in combination with independently explosive "fuels" such as guanidine nitrate,[19][20] as a cheaper (but less stable) alternative to 5-aminotetrazole in the inflators of airbags manufactured by Takata Corporation, which were recalled as unsafe after killing 14 people.[21]
Safety, handling, and storage
Numerous safety guidelines are available for storing and handling ammonium nitrate. Health and safety data are shown on the safety data sheets available from suppliers and from various governments.[22][23][24]
Pure ammonium nitrate does not burn, but as a strong oxidizer, it supports and accelerates the combustion of organic (and some inorganic) material.[22][25][26] It should not be stored near combustible substances.
While ammonium nitrate is stable at ambient temperature and pressure under many conditions, it may detonate from a strong initiation charge. It should not be stored near high explosives or blasting agents.
Molten ammonium nitrate is very sensitive to shock and detonation, particularly if it becomes contaminated with incompatible materials such as combustibles, flammable liquids, acids, chlorates, chlorides, sulfur, metals, charcoal and sawdust.[27][22]
Contact with certain substances such as chlorates, mineral acids and metal sulfides, can lead to vigorous or even violent decomposition capable of igniting nearby combustible material or detonating.[28][29]
Ammonium nitrate begins decomposition after melting, releasing NOx, HNO3, NH
3 and H2O. It should not be heated in a confined space.[22] The resulting heat and pressure from decomposition increases the sensitivity to detonation and increases the speed of decomposition. Detonation may occur at 80 atmospheres. Contamination can reduce this to 20 atmospheres.[27]
Ammonium nitrate has a critical relative humidity of 59.4% at 30°C. At higher humidity it will absorb moisture from the atmosphere. Therefore, it is important to store ammonium nitrate in a tightly sealed container. Otherwise, it can coalesce into a large, solid mass. Ammonium nitrate can absorb enough moisture to liquefy. Blending ammonium nitrate with certain other fertilizers can lower the critical relative humidity.[30]
The potential for use of the material as an explosive has prompted regulatory measures. For example, in Australia, the Dangerous Goods Regulations came into effect in August 2005 to enforce licensing in dealing with such substances.[31] Licenses are granted only to applicants (industry) with appropriate security measures in place to prevent any misuse.[32] Additional uses such as education and research purposes may also be considered, but individual use will not. Employees of those with licenses to deal with the substance are still required to be supervised by authorized personnel and are required to pass a security and national police check before a license may be granted.
Health hazards
Ammonium nitrate is not hazardous to health and is usually used in fertilizer products.[33][34][35]
Ammonium nitrate has an LD50 of 2217 mg/kg,[36] which for comparison is about two-thirds that of table salt.
Disasters
Ammonium nitrate decomposes, non-explosively, into the gases nitrous oxide and water vapor when heated. However, it can be induced to decompose explosively by detonation.[37] Large stockpiles of the material can also be a major fire risk due to their supporting oxidation, a situation which can easily escalate to detonation. Explosions are not uncommon: relatively minor incidents occur most years, and several large and devastating explosions have also occurred. Examples include the Oppau explosion of 1921 (one of the largest artificial non-nuclear explosions), the Texas City disaster of 1947, the 2015 Tianjin explosions in China, and the 2020 Beirut explosion.[38]
Ammonium nitrate can explode through two mechanisms:
- Shock-to-detonation transition. An explosive charge within or in contact with a mass of ammonium nitrate causes the ammonium nitrate to detonate. Examples of such disasters are Kriewald, Morgan (present-day Sayreville, New Jersey), Oppau, and Tessenderlo.
- Deflagration to detonation transition. The ammonium nitrate explosion results from a fire that spreads into the ammonium nitrate (Texas City, TX; Brest; West, TX; Tianjin; Beirut), or from ammonium nitrate mixing with a combustible material during the fire (Gibbstown, Cherokee, Nadadores). The fire must be confined at least to a degree for successful transition from a fire to an explosion.
See also
- Resource recovery
References
- Pradyot Patnaik. Handbook of Inorganic Chemicals. McGraw-Hill, 2002, ISBN 0-07-049439-8
- Martel, B.; Cassidy, K. (2004). Chemical Risk Analysis: A Practical Handbook. Butterworth–Heinemann. p. 362. ISBN 1-903996-65-1.
- "Hazard Rating Information for NFPA Fire Diamonds". Archived from the original on 17 February 2015. Retrieved 13 March 2015.
- Karl-Heinz Zapp "Ammonium Compounds" in Ullmann's Encyclopedia of Industrial Chemistry 2012, Wiley-VCH, Weinheim. doi:10.1002/14356007.a02_243
- "Ammonium nitrate production by country, 2019 - knoema.com". Knoema. Retrieved 14 August 2020.
- Ammonium nitrate sold by ton as U.S. regulation is stymied. Archived 28 February 2018 at the Wayback Machine – The Dallas Morning News
- "Gwihabaite". www.mindat.org.
- "Niter". www.mindat.org.
- "List of Minerals". www.ima-mineralogy.org. 21 March 2011.
- US 4927617, Villard, Alexandre & Cotonea, Yves, "Process of producing concentrated solutions of ammonium nitrate", published 1990-05-22, assigned to Societe Chimique des Charbonnages S.A.
- Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. p. 469. ISBN 978-0-08-037941-8.
- "The chemistry behind the Beirut explosion".
- Choi, C. S.; Prask, H. J. (1983). "The structure of ND4NO3 phase V by neutron powder diffraction". Acta Crystallographica B. 39 (4): 414–420. doi:10.1107/S0108768183002669.
- Kumar, Pratim (December 2019). "Advances in phase stabilization techniques of AN using KDN and other chemical compounds for preparing green oxidizers". Defence Technology. 15 (6): 949–957. doi:10.1016/j.dt.2019.03.002.
- "Nutrient Content of Fertilizer Materials" (PDF). Archived from the original (PDF) on 24 December 2012. Retrieved 27 June 2012.
- Cook, Melvin A. (1974). The Science of Industrial Explosives. IRECO Chemicals. p. 1. ASIN B0000EGDJT.
- Brook, Tom Vanden. "Afghan bomb makers shifting to new explosives for IEDs". USA TODAY.
- Coxworth, Ben (20 September 2021). "Sunlight and salt water join forces in electricity-free cooling system". New Atlas. Gizmag Pty Ltd. Retrieved 21 September 2021.
- US 5531941, Poole, Donald R., "Process for preparing azide-free gas generant composition", published 1996-07-02, assigned to Automotive Systems Laboratory
- Airbag Compound Has Vexed Takata for Years – The New York Times
- A Cheaper Airbag, and Takata's Road to a Deadly Crisis. – The New York Times
- Chemical Advisory: Safe Storage, Handling, and Management of Ammonium Nitrate United States Environmental Protection Agency
- "Storing and handling ammonium nitrate" (PDF). Archived (PDF) from the original on 4 July 2011. Retrieved 22 March 2006.
- "Ammonium nitrate MSDS". Archived from the original on 18 August 2011. Retrieved 25 January 2012.
- Pradyot Patnaik (2002). Handbook of Inorganic Chemicals. McGraw-Hill. ISBN 0-07-049439-8.
- "Ammonium nitrate". PubChem. Retrieved 6 August 2020.
- "Report for Kooragang Island Update PHA MOD1 Report". Orica Mining Services. 1 April 2012. Retrieved 6 August 2020.
- "Chemical Engineering Transactions" (PDF). Archived from the original (PDF) on 14 April 2016.
- "Ammonium Nitrate". webwiser.nlm.nih.gov. Retrieved 6 August 2020.
- Fertilizers Europe (2006). "Guidance for Compatibility of Fertilizer Blending Materials" (PDF).
- "Dangerous Goods (HCDG) Regulations" (PDF).
- Ammonium Nitrate-Regulating its use, Balancing Access & Protection from "Worksafe Victoria". Archived from the original on 11 March 2011.
- CF Industries. "Ammonium nitrate MSDS" (PDF). Archived from the original (PDF) on 27 March 2014.
- "Chemicalland21 – Ammonium Nitrate". Archived from the original on 10 January 2012.
- "Ammonium Nitrate". Paton Fertilizers Pty Ltd. 2005.
- "Material Safety Data Sheet, Ammonium nitrate MSDS". Archived from the original on 18 August 2011. Retrieved 25 January 2012.
- Chaturvedi, Shalini; Dave, Pragnesh N. (January 2013). "Review on Thermal Decomposition of Ammonium Nitrate". Journal of Energetic Materials. 31 (1): 1–26. Bibcode:2013JEnM...31....1C. doi:10.1080/07370652.2011.573523. S2CID 94427830.
- "Lebanon's president calls for two-week state of emergency in Beirut after blast". Reuters. Beirut. 4 August 2020. Retrieved 4 August 2020.
Aoun, in remarks published on the Presidency Twitter account, said it was "unacceptable" that 2,750 tonnes of ammonium nitrate was stored in a warehouse for six years without safety measures and vowed that those responsible would face the "harshest punishments".
Sources
- Properties: UNIDO and International Fertilizer Development Center (1998), Fertilizer Manual, Kluwer Academic Publishers, ISBN 0-7923-5032-4.
External links
- International Chemical Safety Card 0216
- "Storing and Handling Ammonium Nitrate", United Kingdom Health and Safety Executive publication INDG230 (1986)
- Chemical Advisory: Safe Storage, Handling, and Management of Ammonium Nitrate United States Environmental Protection Agency
- Calculators: surface tensions, and densities, molarities and molalities of aqueous ammonium nitrate
| HNO3 | He | ||||||||||||||||
| LiNO3 | Be(NO3)2 | B(NO 3)− 4 |
RONO2 | NO− 3 NH4NO3 |
HOONO2 | FNO3 | Ne | ||||||||||
| NaNO3 | Mg(NO3)2 | Al(NO3)3 | Si | P | S | ClONO2 | Ar | ||||||||||
| KNO3 | Ca(NO3)2 | Sc(NO3)3 | Ti(NO3)4 | VO(NO3)3 | Cr(NO3)3 | Mn(NO3)2 | Fe(NO3)2 Fe(NO3)3 |
Co(NO3)2 Co(NO3)3 |
Ni(NO3)2 | CuNO3 Cu(NO3)2 |
Zn(NO3)2 | Ga(NO3)3 | Ge | As | Se | BrNO3 | Kr |
| RbNO3 | Sr(NO3)2 | Y(NO3)3 | Zr(NO3)4 | NbO(NO3)3 | Mo | Tc | Ru(NO3)3 | Rh(NO3)3 | Pd(NO3)2 Pd(NO3)4 |
AgNO3 Ag(NO3)2 |
Cd(NO3)2 | In(NO3)3 | Sn(NO3)4 | Sb(NO3)3 | Te | INO3 | Xe(NO3)2 |
| CsNO3 | Ba(NO3)2 | Hf(NO3)4 | TaO(NO3)3 | W | Re | Os | Ir | Pt(NO3)2 Pt(NO3)4 |
Au(NO3)3 | Hg2(NO3)2 Hg(NO3)2 |
TlNO3 Tl(NO3)3 |
Pb(NO3)2 | Bi(NO3)3 BiO(NO3) |
Po(NO3)4 | At | Rn | |
| FrNO3 | Ra(NO3)2 | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og | |
| ↓ | |||||||||||||||||
| La(NO3)3 | Ce(NO3)3 Ce(NO3)4 |
Pr(NO3)3 | Nd(NO3)3 | Pm(NO3)3 | Sm(NO3)3 | Eu(NO3)3 | Gd(NO3)3 | Tb(NO3)3 | Dy(NO3)3 | Ho(NO3)3 | Er(NO3)3 | Tm(NO3)3 | Yb(NO3)3 | Lu(NO3)3 | |||
| Ac(NO3)3 | Th(NO3)4 | PaO2(NO3)3 | UO2(NO3)2 | Np(NO3)4 | Pu(NO3)4 | Am(NO3)3 | Cm(NO3)3 | Bk(NO3)3 | Cf | Es | Fm | Md | No | Lr | |||
