Carbon capture and storage
Carbon capture and storage (CCS) or carbon capture and sequestration[2] is the process of capturing carbon dioxide (CO2) before it enters the atmosphere, transporting it, and storing it (carbon sequestration) for centuries or millennia. Usually the CO2 is captured from large point sources, such as a chemical plant or biomass power plant, and then stored in an underground geological formation. The aim is to prevent the release of CO2 from heavy industry with the intent of mitigating the effects of climate change.[3] CO2 has been injected into geological formations for several decades for enhanced oil recovery and after separation from natural gas, but this has been criticised for producing more emissions when the gas or oil is burned.[4]

Carbon capture and utilization (CCU) and CCS are sometimes discussed collectively as carbon capture, utilization, and sequestration (CCUS). This is because CCS is a relatively expensive process yielding a product which is often too cheap.[5] Hence, carbon capture makes economically more sense where the carbon price is high enough, such as in much of Europe,[4] or when combined with a utilization process where the cheap CO2 can be used to produce high-value chemicals to offset the high costs of capture operations.[6]
CO2 can be captured directly from an industrial source, such as a cement kiln, using a variety of technologies; including absorption, adsorption, chemical looping, membrane gas separation or gas hydration.[7][8] As of 2022, about one thousandth of global CO2 emissions are captured by CCS, and most projects are for fossil gas processing.[4]: 32
Storage of the CO2 is either in deep geological formations, or in the form of mineral carbonates. Pyrogenic carbon capture and storage (PyCCS) is also being researched.[9] Geological formations are currently considered the most promising sequestration sites. The US National Energy Technology Laboratory (NETL) reported that North America has enough storage capacity for more than 900 years worth of CO2 at current production rates.[10] A general problem is that long-term predictions about submarine or underground storage security are very difficult and uncertain, and there is still the risk that some CO2 might leak into the atmosphere.[11][12][13] Despite this, a recent evaluation estimates the risk of substantial leakage to be fairly low.[14][15]
Opponents point out that many CCS projects have failed to deliver on promised emissions reductions.[16] Additionally, opponents argue that carbon capture and storage is only a justification for indefinite fossil fuel usage disguised as marginal emission reductions. One of the most well-known failures is the FutureGen program, partnerships between the US federal government and coal energy production companies which were intended to demonstrate ″clean coal″, but never succeeded in producing any carbon-free electricity from coal.
Capture
Capturing CO2 is most cost-effective at point sources, such as large carbon-based energy facilities, industries with major CO2 emissions (e.g. cement production, steelmaking[17]), natural gas processing, synthetic fuel plants and fossil fuel-based hydrogen production plants. Extracting CO2 from air is possible,[18] although the lower concentration of CO2 in air compared to combustion sources complicates the engineering and makes the process therefore more expensive.[19]
Impurities in CO2 streams, like sulfurs and water, can have a significant effect on their phase behavior and could pose a significant threat of increased pipeline and well corrosion. In instances where CO2 impurities exist, especially with air capture, a scrubbing separation process is needed to initially clean the flue gas.[20] It is possible to capture approximately 65% of CO2 embedded in it and sequester it in a solid form.[21]
Broadly, three different technologies exist: post-combustion, pre-combustion, and oxyfuel combustion:
- In post combustion capture, the CO2 is removed after combustion of the fossil fuel—this is the scheme that would apply to fossil-fuel power plants. CO2 is captured from flue gases at power stations or other point sources. The technology is well understood and is currently used in other industrial applications, although at smaller scale than required in a commercial scale station. Post combustion capture is most popular in research because fossil fuel power plants can be retrofitted to include CCS technology in this configuration.[22]
- The technology for pre-combustion is widely applied in fertilizer, chemical, gaseous fuel (H2, CH4), and power production.[23] In these cases, the fossil fuel is partially oxidized, for instance in a gasifier. The CO from the resulting syngas (CO and H2) reacts with added steam (H2O) and is shifted into CO2 and H2. The resulting CO2 can be captured from a relatively pure exhaust stream. The H2 can be used as fuel; the CO2 is removed before combustion. Several advantages and disadvantages apply versus post combustion capture.[24][25] The CO2 is removed after combustion, but before the flue gas expands to atmospheric pressure. The capture before expansion, i.e. from pressurized gas, is standard in almost all industrial CO2 capture processes, at the same scale as required for power plants.[26][27]
- In oxy-fuel combustion[28] the fuel is burned in pure oxygen instead of air. To limit the resulting flame temperatures to levels common during conventional combustion, cooled flue gas is recirculated and injected into the combustion chamber. The flue gas consists of mainly CO2 and water vapour, the latter of which is condensed through cooling. The result is an almost pure CO2 stream. Power plant processes based on oxyfuel combustion are sometimes referred to as "zero emission" cycles, because the CO2 stored is not a fraction removed from the flue gas stream (as in the cases of pre- and post-combustion capture) but the flue gas stream itself. A certain fraction of the CO2 inevitably end up in the condensed water. To warrant the label "zero emission" the water would thus have to be treated or disposed of appropriately.
Separation technologies
The major technologies proposed for carbon capture are:[7][29][30]
- Membrane
- Oxyfuel combustion
- Absorption
- Multiphase absorption
- Adsorption
- Chemical looping combustion
- Calcium looping
- Cryogenic
Absorption, or carbon scrubbing with amines is the dominant capture technology. It is the only carbon capture technology so far that has been used industrially.[31]
CO2 adsorbs to a MOF (Metal–organic framework) through physisorption or chemisorption based on the porosity and selectivity of the MOF leaving behind a CO2 poor gas stream.[32] The CO2 is then stripped off the MOF using temperature swing adsorption (TSA) or pressure swing adsorption (PSA) so the MOF can be reused. Adsorbents and absorbents require regeneration steps where the CO2 is removed from the sorbent or solution that collected it from the flue gas in order for the sorbent or solution to be reused. Monoethanolamine (MEA) solutions, the leading amine for capturing CO2 , have a heat capacity between 3–4 J/g K since they are mostly water.[33][34] Higher heat capacities add to the energy penalty in the solvent regeneration step. Thus, to optimize a MOF for carbon capture, low heat capacities and heats of adsorption are desired. Additionally, high working capacity and high selectivity are desirable in order to capture as much CO2 as possible. However, an energy trade off complicates selectivity and energy expenditure.[35] As the amount of CO2 captured increases, the energy, and therefore cost, required to regenerate increases. A drawback of MOF/CCS is the limitation imposed by their chemical and thermal stability.[22] Research is attempting to optimize MOF properties for CCS. Metal reservoirs are another limiting factor.[36]
About two thirds of CCS cost is attributed to capture, making it the limit to CCS deployment. Optimizing capture would significantly increase CCS feasibility since the transport and storage steps of CCS are rather mature.[37]
An alternate method is chemical looping combustion (CLC). Looping uses a metal oxide as a solid oxygen carrier. Metal oxide particles react with a solid, liquid or gaseous fuel in a fluidized bed combustor, producing solid metal particles and a mixture of CO2 and water vapor. The water vapor is condensed, leaving pure CO2 , which can then be sequestered. The solid metal particles are circulated to another fluidized bed where they react with air, producing heat and regenerating metal oxide particles for return to the combustor. A variant of chemical looping is calcium looping, which uses the alternating carbonation and then calcination of a calcium oxide based carrier.[38]
A 2019 study found CCS plants to be less effective than renewable electricity. The electrical energy returned on energy invested (EROEI) ratios of both production methods were estimated, accounting for their operational and infrastructural energy costs. Renewable electricity production included solar and wind with sufficient energy storage, plus dispatchable electricity production. Thus, rapid expansion of scalable renewable electricity and storage would be preferable over fossil-fuel with CCS. The study did not consider whether both options could be pursued in parallel.[39]
In 2021 High Hopes proposed using high-altitude balloons to capture CO2 cryogenically, using hydrogen to lower the already low-temperature atmosphere sufficiently to produce dry ice that is returned to earth for sequestration.[40]
In sorption enhanced water gas shift (SEWGS) technology a pre-combustion carbon capture process, based on solid adsorption, is combined with the water gas shift reaction (WGS) in order to produce a high pressure hydrogen stream.[41] The CO2 stream produced can be stored or used for other industrial processes.[42]
Transport
After capture, the CO2 must be transported to suitable storage sites. Pipelines are the cheapest form of transport. Ships can be utilized where pipelines are infeasible, and for long enough distances ships may be cheaper than a pipeline.[43] These methods are used for transporting CO2 for other applications. Rail and tanker truck cost about twice as much as pipelines or ships.[43]
For example, approximately 5,800 km of CO2 pipelines operated in the US in 2008, and a 160 km pipeline in Norway,[44] used to transport CO2 to oil production sites where it is injected into older fields to extract oil. This injection is called enhanced oil recovery. Pilot programs are in development to test long-term storage in non-oil producing geologic formations. In the United Kingdom, the Parliamentary Office of Science and Technology envisages pipelines as the main UK transport.[44]
In 2021, two companies, namely Navigator CO2 Ventures and Summit Carbon Solutions were planning pipelines through the Midwestern US from North Dakota to Illinois to connect ethanol companies to sites where liquefied CO2 is injected into porous rock.[45]
Sequestration (storage)
Various approaches have been conceived for permanent storage. These include gaseous storage in deep geological formations (including saline formations and exhausted gas fields), and solid storage by reaction of CO2 with metal oxides to produce stable carbonates. It was once suggested that CO2 could be stored in the oceans, but this would exacerbate ocean acidification and was banned under the London and OSPAR conventions.[46][47]
Geological storage
Geo-sequestration, involves injecting CO2 , generally in supercritical form, into underground geological formations. Oil fields, gas fields, saline formations, unmineable coal seams, and saline-filled basalt formations have been suggested as alternatives. Physical (e.g., highly impermeable caprock) and geochemical trapping mechanisms prevent the CO2 from escaping to the surface.[48]
Unmineable coal seams can be used because CO2 molecules attach to the coal surface. Technical feasibility depends on the coal bed's permeability. In the process of absorption the coal releases previously absorbed methane, and the methane can be recovered (enhanced coal bed methane recovery). Methane revenues can offset a portion of the cost, although burning the resultant methane, however, produces another stream of CO2 to be sequestered.
Saline formations contain mineralized brines and have yet to produce benefit to humans. Saline aquifers have occasionally been used for storage of chemical waste in a few cases. The main advantage of saline aquifers is their large potential storage volume and their ubiquity. The major disadvantage of saline aquifers is that relatively little is known about them. To keep the cost of storage acceptable, geophysical exploration may be limited, resulting in larger uncertainty about the aquifer structure. Unlike storage in oil fields or coal beds, no side product offsets the storage cost. Trapping mechanisms such as structural trapping, residual trapping, solubility trapping and mineral trapping may immobilize the CO2 underground and reduce leakage risks.[48] [49]
Enhanced oil recovery
CO2 is occasionally injected into an oil field as an enhanced oil recovery technique,[50] but because CO2 is released when the oil is burned,[51] it is not carbon neutral.[52]
Algae/bacteria
CO2 can be physically supplied to algae or bacteria that could degrade the CO2. It would ultimately be ideal to exploit CO2 metabolizing bacterium Clostridium thermocellum.[53][54]
Mineral storage
CO2 can exothermically react with metal oxides, which in turn produce stable carbonates (e.g. calcite, magnesite). This process (CO2-to-stone) occurs naturally over periods of years and is responsible for much surface limestone. Olivine is one such MOX.[55] The reaction rate can be accelerated with a catalyst[56] or by increasing temperatures and/or pressures, or by mineral pre-treatment, although this method can require additional energy. The IPCC estimates that a power plant equipped with CCS using mineral storage would need 60–180% more energy than one without.[43] Theoretically, up to 22% of crustal mineral mass is able to form carbonates.
| Earthen oxide | Percent of crust | Carbonate | Enthalpy change (kJ/mol) |
|---|---|---|---|
| SiO2 | 59.71 | ||
| Al2O3 | 15.41 | ||
| CaO | 4.90 | CaCO3 | −179 |
| MgO | 4.36 | MgCO3 | −118 |
| Na2O | 3.55 | Na2CO3 | −322 |
| FeO | 3.52 | FeCO3 | −85 |
| K2O | 2.80 | K2CO3 | −393.5 |
| Fe2O3 | 2.63 | FeCO3 | 112 |
| 21.76 | All carbonates |
Ultramafic mine tailings are a readily available source of fine-grained metal oxides that can serve this purpose.[57] Accelerating passive CO2 sequestration via mineral carbonation may be achieved through microbial processes that enhance mineral dissolution and carbonate precipitation.[58][59][60]
Cost
Cost is a significant factor affecting CCS. The cost of CCS, plus any subsidies, must be less than the expected cost of emitting CO2 for a project to be considered economically favorable.
CCS technology is expected to use between 10 and 40 percent of the energy produced by a power station.[61][62] Energy for CCS is called an energy penalty. It has been estimated that about 60% of the penalty originates from the capture process, 30% comes from compression of CO2 , while the remaining 10% comes from pumps and fans.[63] CCS would increase the fuel requirement of a plant with CCS by about 15% (gas plant).[43] The cost of this extra fuel, as well as storage and other system costs, are estimated to increase the costs of energy from a power plant with CCS by 30–60%.
Constructing CCS units is capital intensive. The additional costs of a large-scale CCS demonstration project are estimated to be €0.5–1.1 billion per project over the project lifetime. Other applications are possible. CCS trials for coal-fired plants in the early 21st century were economically unviable in most countries,[64] including China,[65] in part because revenue from enhanced oil recovery collapsed with the 2020 oil price collapse.[66] A carbon price of at least 100 euros per tonne CO2 is estimated to be needed to make industrial CCS viable,[67] together with carbon tariffs.[68] But, as of mid-2022, the EU Allowance had never reached that price and the Carbon Border Adjustment Mechanism had not yet been implemented.[69] However a company making small modules claims it can get well below that price by mass production by 2022.[70]
According to UK government estimates made in the late 2010s, carbon capture (without storage) is estimated to add 7 GBP per MWh by 2025 to the cost of electricity from a gas-fired power plant: however most CO2 will need to be stored so in total the increase in cost for gas or biomass generated electricity is around 50%.[71]
Business models
Possible business models for industrial carbon capture include:[72]
- Contract for Difference CfDC CO2 certificate strike price
- Cost Plus open book
- Regulated Asset Base (RAB)
- Tradeable tax credits for CCS
- Tradeable CCS certificates + obligation
- Creation of low carbon market
Governments have provided various types of funding for CCS demonstration projects, including tax credits, allocations and grants.[73]
Clean Development Mechanism
One alternative could be through the Clean Development Mechanism of the Kyoto Protocol. At COP16 in 2010, The Subsidiary Body for Scientific and Technological Advice, at its thirty-third session, issued a draft document recommending the inclusion of CCS in geological formations in Clean Development Mechanism project activities.[74] At COP17 in Durban, a final agreement was reached enabling CCS projects to receive support through the Clean Development Mechanism.[75]
Environmental effects
Alkaline solvents
CO2 can be captured with alkaline solvents at low temperatures in the absorber and released CO2 at higher temperatures in a desorber. Chilled ammonia CCS plants emit ammonia. "Functionalized Ammonia" emits less ammonia, but amines may form secondary amines that emit volatile nitrosamines[76] by a side reaction with nitrogen dioxide, which is present in any flue gas. Alternative amines with little to no vapor pressure can avoid these emissions. Nevertheless, practically 100% of remaining sulfur dioxide from the plant is washed out of the flue gas, along with dust/ash.
Natural gas processing and enhanced oil recovery
The Institute for Energy Economics & Financial Analysis has criticised companies for not reporting greenhouse gas emissions from the use of their products.[4]: 33 CO2 from natural-gas processing is often used for EOR.[4] It has been suggested that enhanced oil recovery only be allowed to use anthropogenic CO2 and should only receive financial incentives such as tax credits when carbon negative, which is generally only in the early years of a project.[77]
Gas and coal-fired power plants
Although the global total CO2 emitted by fossil fuel power plants is very large, coal plant flue gas typically only contains 10–14% CO2, and gas power plants only 4–5% CO2.[4]: 37 Cost per tonne CO2 increases as the capacity factor decreases (the plant is used less - for example only for times of highest demand or in emergencies).[4]: 42
The extra energy requirements deriving from CCS for natural gas combined cycle (NGCC) plants range from 11 to 22%.[78] Fuel use and environmental problems (e.g., methane emissions) arising from gas extraction increase accordingly. Plants equipped with selective catalytic reduction systems for nitrogen oxides produced during combustion[79] require proportionally greater amounts of ammonia.
A 2020 study concluded that half as much CCS might be installed in coal-fired plants as in gas-fired: these would be mainly in China and India.[80] However a 2022 study concluded that it would be too expensive for coal power in China.[81]
For super-critical pulverized coal (PC) plants, CCS' energy requirements range from 24 to 40%, while for coal-based gasification combined cycle (IGCC) systems it is 14–25%.[78] Fuel use and environmental problems arising from coal extraction increase accordingly. Plants equipped with flue-gas desulfurization (FGD) systems for sulfur dioxide control require proportionally greater amounts of limestone, and systems equipped with selective catalytic reduction systems for nitrogen oxides produced during combustion require proportionally greater amounts of ammonia. As of 2022 Boundary Dam is the only coal-fired power station which uses post-combustion CCS.[4]: 42
Leakage
Long-term retention
IPCC estimates that leakage risks at properly managed sites are comparable to those associated with current hydrocarbon activity. It recommends that limits be set to the amount of leakage that can take place.[82] However, this finding is contested given the lack of experience.[83][84] CO2 could be trapped for millions of years, and although some leakage may occur, appropriate storage sites are likely to retain over 99% for over 1000 years.[85]
Mineral storage is not regarded as presenting any leakage risks.[86]
Norway's Sleipner gas field is the oldest industrial scale retention project. An environmental assessment conducted after ten years of operation concluded that geosequestration was the most definite form of permanent geological storage method:
Available geological information shows absence of major tectonic events after the deposition of the Utsira formation [saline reservoir]. This implies that the geological environment is tectonically stable and a site suitable for CO2 storage. The solubility trapping [is] the most permanent and secure form of geological storage.[87]
In March 2009 StatoilHydro issued a study documenting the slow spread of CO2 in the formation after more than 10 years operation.[88]
Gas leakage into the atmosphere may be detected via atmospheric gas monitoring, and can be quantified directly via eddy covariance flux measurements.[89][90][91]
Sudden leakage hazards
Transmission pipelines may leak or rupture. Pipelines can be fitted with remotely controlled valves that can limit the release quantity to one pipe section. For example, a severed 19" pipeline section 8 km long could release its 1,300 tonnes in about 3–4 min.[92] At the storage site, the injection pipe can be fitted with non-return valves to prevent an uncontrolled release from the reservoir in case of upstream pipeline damage.
Large-scale releases present asphyxiation risk. In the 1953 Menzengraben mining accident, several thousand tonnes were released and asphyxiated a person 300 meters away.[92] Malfunction of a CO2 industrial fire suppression system in a large warehouse released 50 t CO2 after which 14 people collapsed on the nearby public road.[92] In the Berkel en Rodenrijs incident in December 2008 a modest release from a pipeline under a bridge killed some ducks sheltering there.[93]
Monitoring
Monitoring allows leak detection with enough warning to minimize the amount lost, and to quantify the leak size. Monitoring can be done at both the surface and subsurface levels.[94]
Subsurface
Subsurface monitoring can directly and/or indirectly track the reservoir's status. One direct method involves drilling deep enough to collect a sample. This drilling can be expensive due to the rock's physical properties. It also provides data only at a specific location.
One indirect method sends sound or electromagnetic waves into the reservoir which reflects back for interpretation. This approach provides data over a much larger region; although with less precision.
Both direct and indirect monitoring can be done intermittently or continuously.[94]
Seismic
Seismic monitoring is a type of indirect monitoring. It is done by creating seismic waves either at the surface using a seismic vibrator, or inside a well using a spinning eccentric mass. These waves propagate through geological layers and reflect back, creating patterns that are recorded by seismic sensors placed on the surface or in boreholes.[95] It can identify migration pathways of the CO2 plume.[96]
Examples of seismic monitoring of geological sequestration are the Sleipner sequestration project, the Frio CO2 injection test and the CO2CRC Otway Project.[97] Seismic monitoring can confirm the presence of CO2 in a given region and map its lateral distribution, but is not sensitive to the concentration.
Tracer
Organic chemical tracers, using no radioactive nor Cadmium components, can be used during the injection phase in a CCS project where CO2 is injected into an existing oil or gas field, either for EOR, pressure support or storage. Tracers and methodologies are compatible with CO2 – and at the same time unique and distinguishable from the CO2 itself or other molecules present in the sub-surface. Using laboratory methodology with an extreme detectability for tracer, regular samples at the producing wells will detect if injected CO2 has migrated from the injection point to the producing well. Therefore, a small tracer amount is sufficient to monitor large scale subsurface flow patterns. For this reason, tracer methodology is well-suited to monitor the state and possible movements of CO2 in CCS projects. Tracers can therefore be an aid in CCS projects by acting as an assurance that CO2 is contained in the desired location sub-surface. In the past, this technology has been used to monitor and study movements in CCS projects in Algeria (Mathieson et al. “In Salah CO 2 Storage JIP: CO 2 sequestration monitoring and verification technologies applied at Krechba, Algeria”, Energy Procedia 4:3596-3603), in the Netherlands (Vandeweijer et al. “Monitoring the CO2 injection site: K12B”, Energy Procedia 4 (2011) 5471–5478) as well as in Norway (Snøhvit).
Surface
Eddy covariance is a surface monitoring technique that measures the flux of CO2 from the ground's surface. It involves measuring CO2 concentrations as well as vertical wind velocities using an anemometer.[98] This provides a measure of the vertical CO2 flux. Eddy covariance towers could potentially detect leaks, after accounting for the natural carbon cycle, such as photosynthesis and plant respiration. An example of eddy covariance techniques is the Shallow Release test.[99] Another similar approach is to use accumulation chambers for spot monitoring. These chambers are sealed to the ground with an inlet and outlet flow stream connected to a gas analyzer.[94] They also measure vertical flux. Monitoring a large site would require a network of chambers.
InSAR
InSAR monitoring involves a satellite sending signals down to the Earth's surface where it is reflected back to the satellite's receiver. The satellite is thereby able to measure the distance to that point.[100] CO2 injection into deep sublayers of geological sites creates high pressures. These layers affect layers above and below them, change the surface landscape. In areas of stored CO2 , the ground's surface often rises due to the high pressures. These changes correspond to a measurable change in the distance from the satellite.[100]
Carbon capture and utilization (CCU)
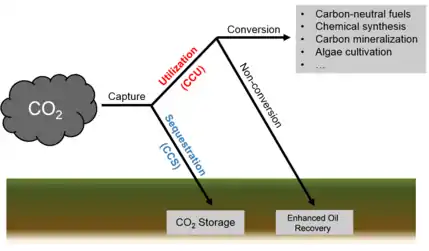
Carbon capture and utilization (CCU) is the process of capturing carbon dioxide (CO2) to be recycled for further usage.[101] Carbon capture and utilization may offer a response to the global challenge of significantly reducing greenhouse gas emissions from major stationary (industrial) emitters.[102] CCU differs from carbon capture and storage (CCS) in that CCU does not aim nor result in permanent geological storage of carbon dioxide. Instead, CCU aims to convert the captured carbon dioxide into more valuable substances or products; such as plastics, concrete or biofuel; while retaining the carbon neutrality of the production processes.
Captured CO2 can be converted to several products: one group being alcohols, such as methanol, to use as biofuels and other alternative and renewable sources of energy. Other commercial products include plastics, concrete and reactants for various chemical synthesis.[103]
Although CCU does not result in a net carbon positive to the atmosphere, there are several important considerations to be taken into account. Because CO2 is a thermodynamically stable form of carbon manufacturing products from it is energy intensive.[104] The availability of other raw materials to create a product should also be considered before investing in CCU.
Considering the different potential options for capture and utilization, research suggests that those involving chemicals, fuels and microalgae have limited potential for CO2 removal, while those that involve construction materials and agricultural use can be more effective.[105]
The profitability of CCU depends partly on the carbon price of CO2 being released into the atmosphere.Social acceptance
Multiple studies indicate that risk and benefit perception are the most essential components of social acceptance.[106]
Risk perception is mostly related to the concerns on its safety issues in terms of hazards from its operations and the possibility of CO2 leakage which may endanger communities, commodities, and the environment in the vicinity of the infrastructure.[107] Other perceived risks relate to tourism and property values.[106]
People who are already affected by climate change, such as drought,[108] tend to be more supportive of CCS. Locally, communities are sensitive to economic factors, including job creation, tourism or related investment.[106]
Experience is another relevant feature. Several field studies concluded that people already involved or used to industry are likely to accept the technology. In the same way, communities who have been negatively affected by any industrial activity are also less supportive of CCS.[106]
Few members of the public know about CCS. This can allow misconceptions that lead to less approval. No strong evidence links knowledge of CCS and public acceptance. However, one study found that communicating information about monitoring tends to have a negative impact on attitudes.[109] Conversely, approval seems to be reinforced when CCS is compared to natural phenomena.[106]
Due to the lack of knowledge, people rely on organizations that they trust. In general, non-governmental organizations and researchers experience higher trust than stakeholders and governments. Opinions amongst NGOs are mixed.[110][111] Moreover, the link between trust and acceptance is at best indirect. Instead, trust has an influence on the perception of risks and benefits.[106]
CCS is embraced by the shallow ecology worldview,[112] which promotes the search for solutions to the effects of climate change in lieu of/in addition to addressing the causes. This involves the use of advancing technology and CCS acceptance is common among techno-optimists. CCS is an "end-of-pipe" solution[106] that reduces atmospheric CO2, instead of minimizing the use of fossil fuel.[106][112]
On 21 January 2021, Elon Musk announced he was donating $100m for a prize for best carbon capture technology.[113]
Environmental justice
Carbon capture facilities are often designed to be located near existing oil and gas infrastructure.[114]
A 2021 DeSmog Blog story highlighted, "CCS hubs are likely be sites in communities already being impacted by the climate crisis like Lake Charles and those along the Mississippi River corridor, where most of the state carbon pollution is emitted from fossil fuel power plants. Exxon, for example, is backing a carbon storage project in Houston's shipping channel, another environmental justice community."[115]
Political debate
CCS has been discussed by political actors at least since the start of the UNFCCC[116] negotiations in the beginning of the 1990s, and remains a very divisive issue.
Some environmental groups raised concerns over leakage given the long storage time required, comparing CCS to storing radioactive waste from nuclear power stations.[117]
Other controversies arose from the use of CCS by policy makers as a tool to fight climate change. In the IPCC's Sixth Assessment Report in 2022, most pathways to keep the increase of global temperature below 2 °C include the use of negative emission technologies (NETs).[118]
Carbon emission status-quo
Opponents claimed that CCS could legitimize the continued use of fossil fuels, as well obviate commitments on emission reduction.
Some examples such as in Norway shows that CCS and other carbon removal technologies gained traction because it allowed the country to pursue its interests regarding the petroleum industry. Norway was a pioneer in emission mitigation, and established a CO2 tax in 1991.[119]
Environmental NGOs
Environmental NGOs are not in widespread agreement about CCS as a potential climate mitigation tool. The main disagreement amid NGOs is whether CCS will reduce CO2 emissions or just perpetuate the use of fossil fuels.[120]
For instance, Greenpeace is strongly against CCS. According to the organization, the use of the technology will keep the world dependent on fossil fuels.[121]
On the other hand, BECCS is used in some IPCC scenarios to help meet mitigation targets.[122] Adopting the IPCC argument that CO2 emissions need to be reduced by 2050 to avoid dramatic consequences, the Bellona Foundation justified CCS as a mitigation action.[121] They claimed fossil fuels are unavoidable for the near term and consequently, CCS is the quickest way to reduce CO2 emissions.[107]
Example projects
According to the Global CCS Institute, in 2020 there was about 40 million tons CO2 per year capacity of CCS in operation and 50 million tons per year in development.[123] In contrast, the world emits about 38 billion tonnes of CO2 every year,[124] so CCS captured about one thousandth of the 2020 CO2 emissions. Iron and steel is expected to dominate industrial CCS in Europe,[5] although there are alternative ways of decarbonizing steel.[125]
CCS and climate change mitigation
CCS can be employed to achieve a number of goals regarding climate change mitigation, such as preventing average global temperature from reaching certain levels above the pre-industrial average. In December 2015, the Paris Agreement articulated a census to not exceed pre-industrial global temperatures by more than 2 °C and recognized that different countries would have different contributions to help realize this goal.[126] Under the Paris Agreement, different scenarios and climate models were analyzed for different temperature goals considering a wide range of mitigation methods from a temperature goal of less than 2 °C to an upper limit of exactly 2 °C increase above the pre-industrial average.
The terms CCS and CCUS (Carbon Capture, Utilization, and Storage) are often used interchangeably. The difference between the two is the specified 'utilization' of the captured carbon and refers to its use for other applications, such as enhanced oil recovery (EOR), potentially making liquid fuel, or the manufacturing of useful consumer goods, such as plastics. Since both approaches capture emitted CO2 and effectively store it, whether that be under-ground in geological formations or long-term trapping in material products, the two terms are often treated the same.
CCS is considered as a basis of one climate stabilization wedge, which is a proposed climate mitigation action to reduce approximately 1 billion tonnes of carbon emissions over 50 years.[127]
CCS and different climate models
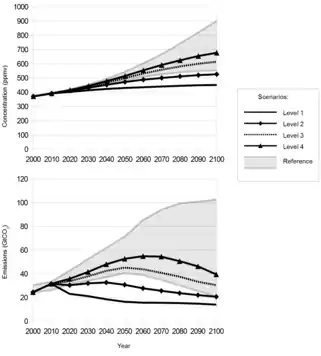
Large scale CCS plays a crucial role in reaching climate change stabilization. According to the IPCC, the carbon emission patterns can greatly vary based on the uncertainty of human power consumption. A file regarding the fluctuations of greenhouse gas emissions is shown to the right. However, CCS' primarily role is to delay the shift from fossil fuels and thereby reducing transition costs. The implementation of default technology assumptions would cost 29-297% more over the century than efforts without CCS for a 430-480 ppm CO2/yr scenario.[128][129] The Paris agreement upholds a goal to reach no more than a 2.0 °C increase above pre-industrial temperatures. If the 2.0 °C goal is to be reached in time, CCS must be utilized to achieve net zero emissions by 2060-2070. After 2060-2070, negative emissions will need to be achieved to remain below the 2.0 °C target. The variations in methods depend heavily on the climate change model being used and the anticipated energy consumption patterns. It is widely agreed upon, however, that CCS would need to be utilized if there is to be any negative climate change mitigation.[130]
CCS and 2.0°C target
The concept of a 2.0 °C came to light in the European Union of 1996 where the goal was to reduce the global temperature range relative pre-industrial levels. The decision of the 2 °C range was decided mostly on the evidence that many ecosystems are at risk if average global temperatures exceeded this limit. In order to limit the anthropogenic emissions such that there is no more than a 2 °C change relative to the periods between 1861 and 1880, carbon emissions would need to be limited to about 1000 GtC by 2100 since that period. However, by the end of 2011 about half of the budget was already released (445 GtC) indicating that a lower budget is necessary.[131]
A distinctive path that aims for a 2.0 °C limit might have complications. The first complication involves the lack of positive feedback loops in IPCC climate models. These loops include reduction of ice sheet size, which would mean less sunlight is reflected and more is absorbed by the darker colored ground or water, and the potential release of greenhouse gases by thawing tundra. Since the lifetime of CO2 in the climate atmosphere is so long, these feedback loops have to be taken into consideration. Another important factor to consider is that a 2.0 °C scenario necessitates tapping into alternative fossil fuels sources that are harder to obtain. Some examples of these methods are the exploitation of tar sands, tar shales, hydrofracking for oil and gas, coal mining, drilling in the Arctic, Amazon, and deep ocean. Therefore, 2.0 °C scenarios result in more CO2 produced per unit of usable energy. Further, the danger of extra released CH4 via mining processes must be taken into account.[132]
Different models are based on when the peak of carbon emissions happen on a global scale. In one article regarding the 2.0 °C scenario with respect to pre-industrial levels, possible approaches are short term and long term emission resolutions as well as the considering the cost effectiveness of different solutions to reduce carbon emissions. Short term goals are set to quantify progress towards the temperature goal. In a short term goal, looking ahead to the year 2020, the allowable carbon emissions must be between 41 and 55 GtCO2 per year. The short term 2 °C scenario is not feasible without CCS.[133]
Currently, greenhouse gas emissions would need to be reduced by 7 Gt of carbon equivalent each year by 2050 to achieve 2 °C stabilization. This requires power generation with CCS at 800 coal-fired power plants of 1 GW energy generation capacity, 180 coal-synfuel plants, or natural gas plants worth 1,600 GW.[134] In this scenario, one of the wedges, or 1 Gt of carbon is accounted for by CCUS.[135]
Achieving below 2.0°C target
A change of temperature below 2 °C is, to certain extent, almost impossible to achieve due to the current carbon emission practices. The IPCC notes that it is difficult to assess a climate mitigation scenario that would limit average global temperature increase to only 1.5 °C above pre-industrial levels. This is mainly due to the fact that few reliable multi-model studies have been conducted to thoroughly explore this scenario. Nevertheless, what few studies that have been done agree that mitigation technologies must be implemented immediately and scaled up quickly and reflect energy demand decrease.[136] A change below 1 °C with respect to pre-industrial era is now inconceivable because by 2017 there was already an increase of 1 °C.[137]
Because of the immediate inability to control the temperature at the 1 °C target, the next realistic target is 1.5 °C. There is enough confidence that past emissions alone (pre-industrial time) will not be enough to go beyond the 1.5 °C target. In other words, if all anthropogenic emissions were stopped today (reduced to zero), any increase beyond the 1 °C change for more than half of a degree before 2100 is unlikely. If anthropogenic emission are considered, the probability for the planet increasing for more than 1.5 °C before 2100 are high. Then, scenarios where the degree change is maintain below 1.5 °C are very challenging to achieve but not impossible.[138]
For a below 2.0 °C target, Shared socioeconomic pathways (SSPs) had been developed adding a socio-economic dimension to the integrative work started by RCPs models. The advantage of using SSPs is that they incorporate social standards, fossil fuel use, geographical development, and high energy demand. SSPs also incorporate the use of six other models such as GCAM4, IMAGE, MESSAGE-GLOBIOM, and REMIND-MAgPIE. The combination of models and scenarios concluded that by 2050, annual CO2 emissions are in the range between 9 and 13 billion tons of CO2. All of the scenarios estimated that temperature will remain below 2.0 °C change with a 66% probability of success. To do so, a 1.9 W/m2 within the year 2100 is necessary. Net zero GHG emissions have to be achieved between 2055 and 2075, and CO2 emissions have to be in a range between 175 and 475 GtCO2 between the years 2016-2100. All SSPs scenarios show a shift away from unabated fossil fuels, that is process without CCS.[138]
Assumptions for below 2.0°C target
To achieve a 1.5 °C target before 2100, the following assumptions have to be considered; emissions have to peak by 2020 and decline after that, it will be necessary to reduce net CO2 emissions to zero and negative emissions have to be a reality by the second half of the 21st century. For this assumptions to take place, CCS has to be implemented in factories that accompany the use of fossil fuels. Because emissions reduction has to be implemented more rigorously for a 1.5 °C target, methods such as BEECS, and natural climate solutions such as afforestation can be used to aim in the reduction of global emissions.[139] BECCS is necessary to achieve a 1.5 °C. It is estimated by the models that with the help of BECCS, between 150 and 12000 GtCO2 still have to be removed from the atmosphere.[138]
Another negative emission strategy which includes CCS can also be approached through DACCS. Direct Air Carbon Capture and Sequestration (DACCS) is a carbon negative technology that utilizes solid amine based capture and it has proven to capture carbon dioxide from the air even though content of the air is much lower than of a flue gas from a coal plant.[140] However, it would require renewable energies to power since approximately 400kJ of work is needed per mole of CO2 capture. Furthermore, it is estimated that the total system cost is $1,000 per tonne of CO2, according to an economic and energetic analysis from 2011.[141]
Going forward in the utilization of models such as SSPss and RCP, feasibility of the model has to be to take into consideration. Feasibility includes concerns in various fields, such as geophysics, technology, economics, social acceptance, and politics, all of which can serve to facilitate or obstruct the carbon capture and sequestration of emissions needed in order to achieve the global temperature targets. Uncertainty in feasibility is especially a problem with more strict temperatures limits such as 1.5 °C. Real world feasibility of SSPs models, or any other models, in general are coarse approximations of reality.[138]
See also
- Bio-energy with carbon capture and storage
- Biological pump
- Biosequestration
- CCS and climate change mitigation
- Carbon capture and storage (timeline)
- Carbon dioxide removal
- Carbon sequestration
- Carbon sink
- Carbon storage in the North Sea
- Climate engineering
- Coal pollution mitigation
- Eddy covariance
- Exhaust gas
- Flue gas
- Flue-gas desulfurization
- Flue-gas stack
- Integrated gasification combined cycle
- Life-cycle greenhouse-gas emissions of energy sources
- Limnic eruption
- Low-carbon economy
- Methane pyrolysis
- North East of England Process Industry Cluster
- Solid sorbents for carbon capture
References
- Abdulla, Ahmed; Hanna, Ryan; Schell, Kristen R.; Babacan, Oytun; et al. (29 December 2020). "Explaining successful and failed investments in U.S. carbon capture and storage using empirical and expert assessments". Environmental Research Letters. 16 (1): 014036. doi:10.1088/1748-9326/abd19e.
- Fanchi, John R; Fanchi, Christopher J (2016). Energy in the 21st Century. World Scientific Publishing Co Inc. p. 350. ISBN 978-981-314-480-4.
- The UK Carbon Capture Usage and Storage deployment pathway (PDF). BEIS. 2018.
- "The carbon capture crux: Lessons learned". ieefa.org. Retrieved 1 October 2022.
- Ghilotti, Davide (26 September 2022). "High carbon prices spurring Europe's CCS drive | Upstream Online". Upstream Online | Latest oil and gas news. Retrieved 1 October 2022.
- "Dream or Reality? Electrification of the Chemical Process Industries". www.aiche-cep.com. Retrieved 22 August 2021.
- Bui, Mai; Adjiman, Claire S.; Bardow, André; Anthony, Edward J.; Boston, Andy; Brown, Solomon; Fennell, Paul S.; Fuss, Sabine; Galindo, Amparo; Hackett, Leigh A.; Hallett, Jason P.; Herzog, Howard J.; Jackson, George; Kemper, Jasmin; Krevor, Samuel; Maitland, Geoffrey C.; Matuszewski, Michael; Metcalfe, Ian S.; Petit, Camille; Puxty, Graeme; Reimer, Jeffrey; Reiner, David M.; Rubin, Edward S.; Scott, Stuart A.; Shah, Nilay; Smit, Berend; Trusler, J. P. Martin; Webley, Paul; Wilcox, Jennifer; Mac Dowell, Niall (2018). "Carbon capture and storage (CCS): the way forward". Energy & Environmental Science. 11 (5): 1062–1176. doi:10.1039/C7EE02342A.
- D'Alessandro, Deanna M.; Smit, Berend; Long, Jeffrey R. (16 August 2010). "Carbon Dioxide Capture: Prospects for New Materials". Angewandte Chemie International Edition. 49 (35): 6058–6082. doi:10.1002/anie.201000431. PMID 20652916.
- Werner, C; Schmidt, H-P; Gerten, D; Lucht, W; Kammann, C (1 April 2018). "Biogeochemical potential of biomass pyrolysis systems for limiting global warming to 1.5 °C". Environmental Research Letters. 13 (4): 044036. Bibcode:2018ERL....13d4036W. doi:10.1088/1748-9326/aabb0e.
- "Carbon Storage Program". netl.doe.gov. Retrieved 30 December 2021.
- Phelps, Jack J.C.; Blackford, Jerry C.; Holt, Jason T.; Polton, Jeff A. (July 2015). "Modelling large-scale CO 2 leakages in the North Sea". International Journal of Greenhouse Gas Control. 38: 210–220. doi:10.1016/j.ijggc.2014.10.013.
- Climatewire, Christa Marshall. "Can Stored Carbon Dioxide Leak?". Scientific American. Retrieved 20 May 2022.
- Vinca, Adriano; Emmerling, Johannes; Tavoni, Massimo (2018). "Bearing the Cost of Stored Carbon Leakage". Frontiers in Energy Research. 6. doi:10.3389/fenrg.2018.00040.
- Alcalde, Juan; Flude, Stephanie; Wilkinson, Mark; Johnson, Gareth; Edlmann, Katriona; Bond, Clare E.; Scott, Vivian; Gilfillan, Stuart M. V.; Ogaya, Xènia; Haszeldine, R. Stuart (12 June 2018). "Estimating geological CO2 storage security to deliver on climate mitigation". Nature Communications. 9 (1): 2201. Bibcode:2018NatCo...9.2201A. doi:10.1038/s41467-018-04423-1. PMC 5997736. PMID 29895846. S2CID 48354961.
- Alcade, Juan; Flude, Stephanie. "Carbon capture and storage has stalled needlessly – three reasons why fears of CO₂ leakage are overblown". The Conversation. Retrieved 20 May 2022.
- Groom, Nichola (7 August 2020). "Problems plagued U.S. CO2 capture project before shutdown: document". Reuters. Retrieved 19 July 2021.
- De Ras, Kevin; Van de Vijver, Ruben; Galvita, Vladimir V; Marin, Guy B; Van Geem, Kevin M (1 December 2019). "Carbon capture and utilization in the steel industry: challenges and opportunities for chemical engineering". Current Opinion in Chemical Engineering. 26: 81–87. doi:10.1016/j.coche.2019.09.001. S2CID 210619173.
- "Capturing CO2 From Air" (PDF). Archived from the original (PDF) on 5 March 2016. Retrieved 29 March 2011.
- "Direct Air Capture Technology (Technology Fact Sheet), Geoengineering Monitor". May 2018. Archived from the original on 26 August 2019. Retrieved 1 July 2018.
- "Good plant design and operation for onshore carbon capture installations and onshore pipelines - 5 CO2 plant design". Energy Institute. Archived from the original on 15 October 2013. Retrieved 13 March 2012.
- "Wallula Energy Resource Center". Wallulaenergy.com. 14 June 2007. Archived from the original on 15 July 2010. Retrieved 2 April 2010.
- Sumida, Kenji; Rogow, David L.; Mason, Jarad A.; McDonald, Thomas M.; Bloch, Eric D.; Herm, Zoey R.; Bae, Tae-Hyun; Long, Jeffrey R. (28 December 2011). "CO2 Capture in Metal–Organic Frameworks". Chemical Reviews. 112 (2): 724–781. doi:10.1021/cr2003272. PMID 22204561.
- "Gasification Body" (PDF). Archived from the original (PDF) on 27 May 2008. Retrieved 2 April 2010.
- "(IGCC) Integrated Gasification Combined Cycle for Carbon Capture & Storage". Claverton Energy Group. (conference, 24 October, Bath)
- "Carbon Capture and Storage at Imperial College London". Imperial College London.
- Bryngelsson, Mårten; Westermark, Mats (2005). Feasibility study of CO2 removal from pressurized flue gas in a fully fired combined cycle: the Sargas project. Proceedings of the 18th International Conference on Efficiency, Cost, Optimization, Simulation and Environmental Impact of Energy Systems. pp. 703–10.
- Bryngelsson, Mårten; Westermark, Mats (2009). "CO2 capture pilot test at a pressurized coal fired CHP plant". Energy Procedia. 1: 1403–10. doi:10.1016/j.egypro.2009.01.184.
- Sweet, William (2008). "Winner: Clean Coal - Restoring Coal's Sheen". IEEE Spectrum. 45: 57–60. doi:10.1109/MSPEC.2008.4428318. S2CID 27311899.
- Jensen, Mark J.; Russell, Christopher S.; Bergeson, David; Hoeger, Christopher D.; Frankman, David J.; Bence, Christopher S.; Baxter, Larry L. (November 2015). "Prediction and validation of external cooling loop cryogenic carbon capture (CCC-ECL) for full-scale coal-fired power plant retrofit". International Journal of Greenhouse Gas Control. 42: 200–212. doi:10.1016/j.ijggc.2015.04.009.
- Baxter, Larry L; Baxter, Andrew; Bever, Ethan; Burt, Stephanie; Chamberlain, Skyler; Frankman, David; Hoeger, Christopher; Mansfield, Eric; Parkinson, Dallin; Sayre, Aaron; Stitt, Kyler (28 September 2019). "Cryogenic Carbon Capture Development Final/Technical Report": DOE–SES–28697, 1572908. doi:10.2172/1572908. OSTI 1572908. S2CID 213628936.
{{cite journal}}: Cite journal requires|journal=(help) - "Facility Data - Global CCS Institute". co2re.co. Retrieved 17 November 2020.
- "MOFs for CO2". MOF Technologies. Retrieved 7 April 2021.
- Herm, Zoey R.; Swisher, Joseph A.; Smit, Berend; Krishna, Rajamani; Long, Jeffrey R. (20 April 2011). "Metal−Organic Frameworks as Adsorbents for Hydrogen Purification and Precombustion CO2 Capture" (PDF). Journal of the American Chemical Society. 133 (15): 5664–5667. doi:10.1021/ja111411q. PMID 21438585.
- Kulkarni, Ambarish R.; Sholl, David S. (18 June 2012). "Analysis of Equilibrium-Based TSA Processes for Direct Capture of CO2 from Air". Industrial & Engineering Chemistry Research. 51 (25): 8631–8645. doi:10.1021/ie300691c.
- Millward, Andrew R.; Yaghi, Omar M. (December 2005). "Metal−Organic Frameworks with Exceptionally High Capacity for Storage of CO2 at Room Temperature". Journal of the American Chemical Society. 127 (51): 17998–17999. doi:10.1021/ja0570032. PMID 16366539.
- Smit, Berend; Reimer, Jeffrey R.; Oldenburg, Curtis M.; Bourg, Ian C. (2014). Introduction to Carbon Capture and Sequestration. Imperial College Press. ISBN 978-1-78326-327-1.
- McDonald, Thomas M.; Mason, Jarad A.; Kong, Xueqian; Bloch, Eric D.; Gygi, David; Dani, Alessandro; Crocellà, Valentina; Giordanino, Filippo; Odoh, Samuel O.; Drisdell, Walter S.; Vlaisavljevich, Bess; Dzubak, Allison L.; Poloni, Roberta; Schnell, Sondre K.; Planas, Nora; Lee, Kyuho; Pascal, Tod; Wan, Liwen F.; Prendergast, David; Neaton, Jeffrey B.; Smit, Berend; Kortright, Jeffrey B.; Gagliardi, Laura; Bordiga, Silvia; Reimer, Jeffrey A.; Long, Jeffrey R. (11 March 2015). "Cooperative insertion of CO2 in diamine-appended metal-organic frameworks" (PDF). Nature. 519 (7543): 303–308. Bibcode:2015Natur.519..303M. doi:10.1038/nature14327. hdl:11250/2458220. PMID 25762144. S2CID 4447122.
- "The Global Status of CCS: 2011 - Capture". The Global CCS Institute. Archived from the original on 6 February 2013. Retrieved 26 March 2012.
- Sgouridis, Sgouris; Carbajales-Dale, Michael; Csala, Denes; Chiesa, Matteo; Bardi, Ugo (June 2019). "Comparative net energy analysis of renewable electricity and carbon capture and storage" (PDF). Nature Energy. 4 (6): 456–465. Bibcode:2019NatEn...4..456S. doi:10.1038/s41560-019-0365-7. S2CID 134169612.
- Blain, Loz (4 May 2021). "High Hopes claims stratospheric breakthrough in direct air CO2 capture". New Atlas. Archived from the original on 4 May 2021. Retrieved 5 May 2021.
- Jansen, Daniel; van Selow, Edward; Cobden, Paul; Manzolini, Giampaolo; Macchi, Ennio; Gazzani, Matteo; Blom, Richard; Heriksen, Partow Pakdel; Beavis, Rich; Wright, Andrew (1 January 2013). "SEWGS Technology is Now Ready for Scale-up!". Energy Procedia. 37: 2265–2273. doi:10.1016/j.egypro.2013.06.107.
- (Eric) van Dijk, H. A. J.; Cobden, Paul D.; Lukashuk, Liliana; de Water, Leon van; Lundqvist, Magnus; Manzolini, Giampaolo; Cormos, Calin-Cristian; van Dijk, Camiel; Mancuso, Luca; Johns, Jeremy; Bellqvist, David (1 October 2018). "STEPWISE Project: Sorption-Enhanced Water-Gas Shift Technology to Reduce Carbon Footprint in the Iron and Steel Industry". Johnson Matthey Technology Review. 62 (4): 395–402. doi:10.1595/205651318X15268923666410. hdl:11311/1079169. S2CID 139928989.
- [IPCC, 2005] IPCC special report on CO2 Capture and Storage. Prepared by working group III of the Intergovernmental Panel on Climate Change. Metz, B., O. Davidson, H. C. de Coninck, M. Loos, and L.A. Meyer (eds.). Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA, 442 pp. Available in full at www.ipcc.ch Archived 10 February 2010 at the Wayback Machine (PDF - 22.8MB)
- "CO2 Capture, transport and storage" (PDF). Postnote. Parliamentary Office of Science and Technology. 335. June 2009. Retrieved 10 August 2019.
Since 2008 Norway's Statoil has been transporting CO2 (obtained from natural gas extraction) through a 160 km seabed pipeline
- STEPHEN GROVES (24 July 2021). "Carbon-capture pipelines offer climate aid; activists wary". ABC News. Retrieved 17 February 2022.
- Dixon, Tim; Greaves, Andy; Christophersen, Oyvind; Vivian, Chris; Thomson, Jolyon (February 2009). "International marine regulation of CO2 geological storage. Developments and implications of London and OSPAR". Energy Procedia. 1 (1): 4503–4510. doi:10.1016/j.egypro.2009.02.268.
- "CO2 Capture and Storage". www.greenfacts.org. Retrieved 30 December 2021.
- "Good plant design and operation for onshore carbon capture installations and onshore pipelines - Storage". Energy Institute. Archived from the original on 18 September 2012. Retrieved 11 December 2012.
- Edward Hinton and Andrew Woods (2021). "Capillary trapping in a vertically heterogeneous porous layer". J. Fluid Mech. 910: A44. Bibcode:2021JFM...910A..44H. doi:10.1017/jfm.2020.972. S2CID 231636769.
- "November: Whatever happened to enhanced oil recovery?". www.iea.org. Retrieved 17 June 2019.
- Porter, Kathryn (20 July 2018). "Smoke & mirrors: a new report into the viability of CCS". Watt-Logic. Retrieved 17 June 2019.
- "Occidental To Remove CO2 From Air, Use It To Boost Oil Recovery In The Permian". OilPrice.com. Retrieved 17 June 2019.
- Xiong, Wei; Lin, Paul P.; Magnusson, Lauren; Warner, Lisa; Liao, James C.; Maness, Pin-Ching; Chou, Katherine J. (28 October 2016). "CO2-fixing one-carbon metabolism in a cellulose-degrading bacterium Clostridium thermocellum". Proceedings of the National Academy of Sciences. 113 (46): 13180–13185. Bibcode:2016PNAS..11313180X. doi:10.1073/pnas.1605482113. PMC 5135332. PMID 27794122.
- "Mechanical CO2 sequestration improves algae production – Chemical Engineering – Page 1". March 2019. Retrieved 26 March 2019.
- Schuiling, Olaf. "Olaf Schuiling proposes olivine rock grinding". Archived from the original on 11 April 2013. Retrieved 23 December 2011.
- Bhaduri, Gaurav A.; Šiller, Lidija (2013). "Nickel nanoparticles catalyse reversible hydration of CO2 for mineralization carbon capture and storage". Catalysis Science & Technology. 3 (5): 1234. doi:10.1039/C3CY20791A.
- Wilson, Siobhan A.; Dipple, Gregory M.; Power, Ian M.; Thom, James M.; Anderson, Robert G.; Raudsepp, Mati; Gabites, Janet E.; Southam, Gordon (2009). "CO2 Fixation within Mine Wastes of Ultramafic-Hosted Ore Deposits: Examples from the Clinton Creek and Cassiar Chrysotile Deposits, Canada". Economic Geology. 104: 95–112. doi:10.2113/gsecongeo.104.1.95.
- Power, Ian M.; Dipple, Gregory M.; Southam, Gordon (2010). "Bioleaching of Ultramafic Tailings by Acidithiobacillus spp. For CO2 Sequestration". Environmental Science & Technology. 44 (1): 456–62. Bibcode:2010EnST...44..456P. doi:10.1021/es900986n. PMID 19950896.
- Power, Ian M; Wilson, Siobhan A; Thom, James M; Dipple, Gregory M; Southam, Gordon (2007). "Biologically induced mineralization of dypingite by cyanobacteria from an alkaline wetland near Atlin, British Columbia, Canada". Geochemical Transactions. 8: 13. doi:10.1186/1467-4866-8-13. PMC 2213640. PMID 18053262.
- Power, Ian M.; Wilson, Siobhan A.; Small, Darcy P.; Dipple, Gregory M.; Wan, Wankei; Southam, Gordon (2011). "Microbially Mediated Mineral Carbonation: Roles of Phototrophy and Heterotrophy". Environmental Science & Technology. 45 (20): 9061–8. Bibcode:2011EnST...45.9061P. doi:10.1021/es201648g. PMID 21879741.
- Rochon, Emily et al. False Hope: Why carbon capture and storage won't save the climate Archived 4 May 2009 at the Wayback Machine Greenpeace, May 2008, p. 5.
- Thorbjörnsson, Anders; Wachtmeister, Henrik; Wang, Jianliang; Höök, Mikael (April 2015). "Carbon capture and coal consumption: Implications of energy penalties and large scale deployment". Energy Strategy Reviews. 7: 18–28. doi:10.1016/j.esr.2014.12.001.
- Rubin, Edward S.; Mantripragada, Hari; Marks, Aaron; Versteeg, Peter; Kitchin, John (October 2012). "The outlook for improved carbon capture technology". Progress in Energy and Combustion Science. 38 (5): 630–671. doi:10.1016/j.pecs.2012.03.003.
- Keating, Dave (18 September 2019). "'We need this dinosaur': EU lifts veil on gas decarbonisation strategy". euractiv.com. Retrieved 27 September 2019.
- "Carbon Capture, Storage and Utilization to the Rescue of Coal? Global Perspectives and Focus on China and the United States". www.ifri.org. Retrieved 27 September 2019.
- "CCUS in Power – Analysis". IEA. Retrieved 20 November 2020.
- "Call for open debate on CCU and CCS to save industry emissions". Clean Energy Wire. 27 September 2018. Retrieved 17 June 2019.
- Butler, Clark (July 2020). "Carbon Capture and Storage Is About Reputation, Not Economics" (PDF). IEEFA.
- Twidale, Susanna (14 October 2021). "Analysts raise EU carbon price forecasts as gas rally drives up coal power". Reuters. Retrieved 1 November 2021.
- "Scaling Carbon Capture Might Mean Thinking Small, Not Big". Bloomberg.com. 30 October 2021. Retrieved 1 November 2021.
- "Energy" (PDF).
- "Industrial carbon capture business models" (PDF).
- "Global Status of CCS Report:2011". Global CCS Institute. Archived from the original on 12 January 2012. Retrieved 14 December 2011.
- "SBSTA Presents Global CO2 Capture and Storage Data at COP16". Archived from the original on 28 July 2011.
- Bonner, Mark. "CCS enters the CDM at CMP 7". Global CCS Institute. Archived from the original on 24 January 2013. Retrieved 7 May 2012.
- "CCS - Norway: Amines, nitrosamines and nitramines released in Carbon Capture Processes should not exceed 0.3 ng/m3 air (The Norwegian Institute of Public Health) - ekopolitan". www.ekopolitan.com. Archived from the original on 23 September 2015. Retrieved 19 December 2012.
- Roberts, David (2 October 2019). "Could squeezing more oil out of the ground help fight climate change?". Vox. Retrieved 1 October 2022.
- "IPCC Special Report: Carbon Capture and Storage Technical Summary. IPCC. p. 27" (PDF). Archived from the original (PDF) on 1 November 2013. Retrieved 6 October 2013.
- "No, Natural Gas Power Plants Are Not Clean". Union of Concerned Scientists. 9 November 2018. Retrieved 3 October 2020.
- "Powering through the coming energy transition". MIT News | Massachusetts Institute of Technology. Retrieved 20 November 2020.
- Zhuo, Zhenyu; Du, Ershun; Zhang, Ning; Nielsen, Chris P.; Lu, Xi; Xiao, Jinyu; Wu, Jiawei; Kang, Chongqing (December 2022). "Cost increase in the electricity supply to achieve carbon neutrality in China". Nature Communications. 13 (1): 3172. Bibcode:2022NatCo..13.3172Z. doi:10.1038/s41467-022-30747-0. PMC 9177843. PMID 35676273. S2CID 249521236.
- "IPCC Special Report: CO2 Capture and Storage Technical Summary" (PDF). Intergovernmental Panel on Climate Change. Archived from the original (PDF) on 5 October 2011. Retrieved 5 October 2011.
- Viebahn, Peter; Nitsch, Joachim; Fischedick, Manfred; Esken, Andrea; Schüwer, Dietmar; Supersberger, Nikolaus; Zuberbühler, Ulrich; Edenhofer, Ottmar (April 2007). "Comparison of carbon capture and storage with renewable energy technologies regarding structural, economic, and ecological aspects in Germany". International Journal of Greenhouse Gas Control. 1 (1): 121–133. doi:10.1016/S1750-5836(07)00024-2.
- "University of Sydney: Global warming effect of leakage from CO2 storage" (PDF). March 2013.
- "Global Status of BECCS Projects 2010 - Storage Security". Archived from the original on 19 May 2013. Retrieved 5 April 2012.
- "Making Minerals-How Growing Rocks Can Help Reduce Carbon Emissions". www.usgs.gov. Retrieved 31 October 2021.
- Wagner, Leonard (2007). "Carbon Capture and Storage" (PDF). Moraassociates.com. Archived from the original (PDF) on 21 March 2012.
- "Norway: StatoilHydro's Sleipner carbon capture and storage project proceeding successfully". Energy-pedia. 8 March 2009. Retrieved 19 December 2009.
- US DOE, 2012. Best Practices for Monitoring, Verification and Accounting of CO2 Stored in Deep Geologic Formations - 2012 Update.
- Holloway, S., A. Karimjee, M. Akai, R. Pipatti, and K. Rypdal, 2006–2011. CO2 Transport, Injection and Geological Storage, in Eggleston H.S., Buendia L., Miwa K., Ngara T., and Tanabe K. (Eds.), IPCC Guidelines for National Greenhouse Gas Inventories, IPCC National Greenhouse Gas Inventories Programme, WMO/UNEP
- Miles, Natasha L.; Davis, Kenneth J.; Wyngaard, John C. (2005). "Detecting Leaks from Belowground CO2 Reservoirs Using Eddy Covariance". CO2 Capture for Storage in Deep Geologic Formations. Elsevier Science. pp. 1031–1044. doi:10.1016/B978-008044570-0/50149-5. ISBN 978-0-08-044570-0.
- Hedlund, Frank Huess (2012). "The extreme CO2 outburst at the Menzengraben potash mine 7 July 1953" (PDF). Safety Science. 50 (3): 537–53. doi:10.1016/j.ssci.2011.10.004. S2CID 49313927.
- "Eendensterfte door lek in CO2-leiding (Duck deaths from leaking CO2 pipeline)". March 2013. (in Dutch)
- Smit, Berend; Reimer, Jeffery A.; Oldenburg, Curtis M.; Bourg, Ian C. Introduction to Carbon Capture and Sequestration (The Berkeley Lectures on Energy - Vol. 1 ed.). Imperial College Press.
- Biondi, Biondo; de Ridder, Sjoerd; Chang, Jason (2013). 5.2 Continuous passive-seismic monitoring of CO2 geologic sequestration projects (PDF). Stanford University Global Climate and Energy Project 2013 Technical Report (Report). Retrieved 6 May 2016.
- "Review of Offshore Monitoring for CCS Projects". IEAGHG. IEA Greenhouse Gas R&D Programme. Archived from the original on 3 June 2016. Retrieved 6 May 2016.
- Pevzner, Roman; Urosevic, Milovan; Popik, Dmitry; Shulakova, Valeriya; Tertyshnikov, Konstantin; Caspari, Eva; Correa, Julia; Dance, Tess; Kepic, Anton; Glubokovskikh, Stanislav; Ziramov, Sasha; Gurevich, Boris; Singh, Rajindar; Raab, Matthias; Watson, Max; Daley, Tom; Robertson, Michelle; Freifeld, Barry (August 2017). "4D surface seismic tracks small supercritical CO2 injection into the subsurface: CO2CRC Otway Project". International Journal of Greenhouse Gas Control. 63: 150–157. doi:10.1016/j.ijggc.2017.05.008.
- Madsen, Rod; Xu, Liukang; Claassen, Brent; McDermitt, Dayle (February 2009). "Surface Monitoring Method for Carbon Capture and Storage Projects". Energy Procedia. 1 (1): 2161–2168. doi:10.1016/j.egypro.2009.01.281.
- Trautz, Robert C.; Pugh, John D.; Varadharajan, Charuleka; Zheng, Liange; Bianchi, Marco; Nico, Peter S.; Spycher, Nicolas F.; Newell, Dennis L.; Esposito, Richard A.; Wu, Yuxin; Dafflon, Baptiste; Hubbard, Susan S.; Birkholzer, Jens T. (20 September 2012). "Effect of Dissolved CO2 on a Shallow Groundwater System: A Controlled Release Field Experiment". Environmental Science & Technology. 47 (1): 298–305. doi:10.1021/es301280t. PMID 22950750. S2CID 7382685.
- "InSAR—Satellite-based technique captures overall deformation "picture"". USGS Science for a Changing World. US Geological Survey. Retrieved 6 May 2016.
- Cuéllar-Franca, Rosa M.; Azapagic, Adisa (March 2015). "Carbon capture, storage and utilisation technologies: A critical analysis and comparison of their life cycle environmental impacts". Journal of CO2 Utilization. 9: 82–102. doi:10.1016/j.jcou.2014.12.001.
- "Carbon Capture". Center for Climate and Energy Solutions. Retrieved 22 April 2020.
- Dibenedetto, Angela; Angelini, Antonella; Stufano, Paolo (March 2014). "Use of carbon dioxide as feedstock for chemicals and fuels: homogeneous and heterogeneous catalysis: Use of carbon dioxide as feedstock for chemicals and fuels". Journal of Chemical Technology & Biotechnology. 89 (3): 334–353. doi:10.1002/jctb.4229.
- Smit, Berend; Reimer, Jeffrey A; Oldenburg, Curtis M; Bourg, Ian C (18 June 2013). Introduction to Carbon Capture and Sequestration. The Berkeley Lectures on Energy. Imperial College Press. doi:10.1142/p911. ISBN 9781783263271.
- Hepburn, Cameron; Adlen, Ella; Beddington, John; Carter, Emily A.; Fuss, Sabine; Mac Dowell, Niall; Minx, Jan C.; Smith, Pete; Williams, Charlotte K. (6 November 2019). "The technological and economic prospects for CO2 utilization and removal". Nature. 575 (7781): 87–97. Bibcode:2019Natur.575...87H. doi:10.1038/s41586-019-1681-6. PMID 31695213.
- L׳Orange Seigo, Selma; Dohle, Simone; Siegrist, Michael (October 2014). "Public perception of carbon capture and storage (CCS): A review". Renewable and Sustainable Energy Reviews. 38: 848–863. doi:10.1016/j.rser.2014.07.017.
- Agaton, Casper Boongaling (November 2021). "Application of real options in carbon capture and storage literature: Valuation techniques and research hotspots". Science of the Total Environment. 795: 148683. Bibcode:2021ScTEn.795n8683A. doi:10.1016/j.scitotenv.2021.148683. PMID 34246146.
- Anderson, Carmel; Schirmer, Jacki; Abjorensen, Norman (August 2012). "Exploring CCS community acceptance and public participation from a human and social capital perspective". Mitigation and Adaptation Strategies for Global Change. 17 (6): 687–706. doi:10.1007/s11027-011-9312-z. S2CID 153912327.
- L'Orange Seigo, Selma; Wallquist, Lasse; Dohle, Simone; Siegrist, Michael (November 2011). "Communication of CCS monitoring activities may not have a reassuring effect on the public". International Journal of Greenhouse Gas Control. 5 (6): 1674–1679. doi:10.1016/j.ijggc.2011.05.040.
- Anderson, Jason; Chiavari, Joana (February 2009). "Understanding and improving NGO position on CCS". Energy Procedia. 1 (1): 4811–4817. doi:10.1016/j.egypro.2009.02.308.
- Wong-Parodi, Gabrielle; Ray, Isha; Farrell, Alexander E (April 2008). "Environmental non-government organizations' perceptions of geologic sequestration". Environmental Research Letters. 3 (2): 024007. Bibcode:2008ERL.....3b4007W. doi:10.1088/1748-9326/3/2/024007.
- Mulkens, J. (2018). Carbon Capture and Storage in the Netherlands: protecting the growth paradigm?. Localhost (Thesis). hdl:1874/368133.
- @elonmusk (21 January 2021). "Am donating $100M towards a prize for best carbon capture technology" (Tweet) – via Twitter.
- "Denbury Inc - Oil & Gas Operations". www.denbury.com. Retrieved 19 July 2021.
- Dermansky, Julie (8 July 2021). "Environmental Justice Concerns Raised at a Hearing on Louisiana's Bid For Authority to Permit Carbon Capture and Storage Projects". DeSmog. Retrieved 19 July 2021.
- Carton, Wim; Asiyanbi, Adeniyi; Beck, Silke; Buck, Holly J.; Lund, Jens F. (November 2020). "Negative emissions and the long history of carbon removal". WIREs Climate Change. 11 (6). doi:10.1002/wcc.671.
- Simon Robinson (22 January 2012). "Cutting Carbon: Should We Capture and Store It?". Time. Archived from the original on 24 January 2010.
- Hunt, Kara (20 April 2022). "What does the latest IPCC report say about carbon capture?". Clean Air Task Force. Retrieved 1 October 2022.
- Røttereng, Jo-Kristian S. (May 2018). "When climate policy meets foreign policy: Pioneering and national interest in Norway's mitigation strategy". Energy Research & Social Science. 39: 216–225. doi:10.1016/j.erss.2017.11.024.
- Corry, Olaf; Reiner, David (2011). "Evaluating global Carbon Capture and Storage (CCS) communication materials: A survey of global CCS communications" (PDF). CSIRO: 1–46 – via Global CCS Institute.
- Corry, Olaf; Riesch, Hauke (2012). "Beyond 'For Or Against': Environmental NGO-evaluations of CCS as a climate change solution". In Markusson, Nils; Shackley, Simon; Evar, Benjamin (eds.). The Social Dynamics of Carbon Capture and Storage: Understanding CCS Representations, Governance and Innovation. Routledge. pp. 91–110. ISBN 978-1-84971-315-3.
- "Summary for Policymakers — Global Warming of 1.5 ºC". Archived from the original on 31 May 2019. Retrieved 1 June 2019.
- "Global Status Report". Global CCS Institute. Retrieved 31 May 2021.
- "Carbon Capture, Utilisation and Storage: Effects on Climate Change". actionaidrecycling.org.uk. 17 March 2021. Retrieved 31 May 2021.
- "What is net-zero steel and why do we need it?". World Economic Forum. Retrieved 1 October 2022.
- "INDC - Submissions". www4.unfccc.int. Retrieved 2 December 2018.
- "Stabilization Wedges Introduction | Carbon Mitigation Initiative". cmi.princeton.edu. Retrieved 2 December 2018.
- "DOE - Carbon Capture Utilization and Storage_2016!09!07 | Carbon Capture And Storage | Climate Change Mitigation". Scribd. Retrieved 3 December 2018.
- Pye, Steve; Li, Francis G. N.; Price, James; Fais, Birgit (March 2017). "Achieving net-zero emissions through the reframing of UK national targets in the post-Paris Agreement era" (PDF). Nature Energy. 2 (3): 17024. Bibcode:2017NatEn...217024P. doi:10.1038/nenergy.2017.24. ISSN 2058-7546.
- Rogelj, Joeri; Schaeffer, Michiel; Meinshausen, Malte; Knutti, Reto; Alcamo, Joseph; Riahi, Keywan; Hare, William (2015). "Zero emission targets as long-term global goals for climate protection". Environmental Research Letters. 10 (10): 105007. Bibcode:2015ERL....10j5007R. doi:10.1088/1748-9326/10/10/105007. ISSN 1748-9326.
- Intergovernmental Panel on Climate Change, ed. (2014), "Near-term Climate Change: Projections and Predictability" (PDF), Climate Change 2013 - the Physical Science Basis, Cambridge University Press, pp. 953–1028, doi:10.1017/cbo9781107415324.023, ISBN 9781107415324
- Hansen, James; Kharecha, Pushker; Sato, Makiko; Masson-Delmotte, Valerie; Ackerman, Frank; Beerling, David J.; Hearty, Paul J.; Hoegh-Guldberg, Ove; Hsu, Shi-Ling (3 December 2013). "Assessing "Dangerous Climate Change": Required Reduction of Carbon Emissions to Protect Young People, Future Generations and Nature". PLOS ONE. 8 (12): e81648. Bibcode:2013PLoSO...881648H. doi:10.1371/journal.pone.0081648. ISSN 1932-6203. PMC 3849278. PMID 24312568.
- Rogelj, Joeri; McCollum, David L.; O'Neill, Brian C.; Riahi, Keywan (16 December 2012). "2020 emissions levels required to limit warming to below 2 °C". Nature Climate Change. 3 (4): 405–412. doi:10.1038/nclimate1758. ISSN 1758-678X.
- "The Wedge Approach to Climate Change | World Resources Institute". www.wri.org. Retrieved 4 December 2018.
- "Carbon Capture, Utilization, and Storage: Climate Change, Economic Competitiveness, and Energy Security" (PDF). www.energy.gov. U.S. Department of Energy. August 2016. Retrieved 4 December 2018.
- "Intergovernmental Panel on Climate Change (IPCC) Global Surface Warming Scenarios", Multimedia Atlas of Global Warming and Climatology, SAGE Publications Ltd, 2014, doi:10.4135/9781483351384.n48, ISBN 9781483351384
- M. R. Allen, O. P. Dube, W. Solecki, F. Aragón–Durand, W. Cramer, S. Humphreys, M. Kainuma, J. Kala, N. Mahowald, Y. Mulugetta, R. Perez, M. Wairiu, K. Zickfeld, 2018, Framing and Context. In: Global warming of 1.5°C. An IPCC Special Report on the impacts of global warming of 1.5°C above pre-industrial levels and related global greenhouse gas emission pathways, in the context of strengthening the global response to the threat of climate change, sustainable development, and efforts to eradicate poverty [V. Masson-Delmotte, P. Zhai, H. O. Pörtner, D. Roberts, J. Skea, P.R. Shukla, A. Pirani, W. Moufouma-Okia, C. Péan, R. Pidcock, S. Connors, J. B. R. Matthews, Y. Chen, X. Zhou, M. I. Gomis, E. Lonnoy, T. Maycock, M. Tignor, T. Waterfield (eds.)]. In Press.
- Tavoni, Massimo; Stehfest, Elke; Humpenöder, Florian; Havlík, Petr; Harmsen, Mathijs; Fricko, Oliver; Edmonds, Jae; Drouet, Laurent; Doelman, Jonathan (April 2018). "Scenarios towards limiting global mean temperature increase below 1.5 °C" (PDF). Nature Climate Change. 8 (4): 325–332. Bibcode:2018NatCC...8..325R. doi:10.1038/s41558-018-0091-3. hdl:1874/372779. ISSN 1758-6798. S2CID 56238230.
- "New scenarios show how the world could limit warming to 1.5C in 2100". Carbon Brief. 5 March 2018. Retrieved 6 December 2018.
- Choi, Sunho; Drese, Jeffrey H.; Eisenberger, Peter M.; Jones, Christopher W. (15 March 2011). "Application of Amine-Tethered Solid Sorbents for Direct CO2Capture from the Ambient Air". Environmental Science & Technology. 45 (6): 2420–2427. Bibcode:2011EnST...45.2420C. doi:10.1021/es102797w. ISSN 0013-936X. PMID 21323309.
- House, Kurt Zenz; Baclig, Antonio C.; Ranjan, Manya; Nierop, Ernst A. van; Wilcox, Jennifer; Herzog, Howard J. (20 December 2011). "Economic and energetic analysis of capturing CO2 from ambient air". Proceedings of the National Academy of Sciences. 108 (51): 20428–20433. Bibcode:2011PNAS..10820428H. doi:10.1073/pnas.1012253108. ISSN 0027-8424. PMC 3251141. PMID 22143760.
Further reading
- UK Committee on Climate Change (2018). Biomass in a low-carbon economy (PDF).
- Fajardy, Mathilde; Köberle, Alexandre; Mac Dowell, Niall; Fantuzzi, Andrea (2019). "BECCS deployment: a reality check" (PDF). Grantham Institute Imperial College London.
- Stephens, Jennie C. (5 October 2017). "Growing interest in carbon capture and storage (CCS) for climate change mitigation". Sustainability: Science, Practice and Policy. 2 (2): 4–13. doi:10.1080/15487733.2006.11907979.
External links
 Media related to Carbon capture and storage at Wikimedia Commons
Media related to Carbon capture and storage at Wikimedia Commons- DOE Fossil Energy Department of Energy programs in CO2 capture and storage
- US Department of Energy
- US Gulf coast
- Zero Emissions Platform - technical adviser to the EU Commission on the deployment of CCS and CCU
- National Assessment of Geologic CO2 Storage Resources: Results United States Geological Survey
- MIT Carbon Capture and Sequestration