Franck–Hertz experiment
The Franck–Hertz experiment was the first electrical measurement to clearly show the quantum nature of atoms, and thus "transformed our understanding of the world".[1] It was presented on April 24, 1914, to the German Physical Society in a paper by James Franck and Gustav Hertz.[2][3] Franck and Hertz had designed a vacuum tube for studying energetic electrons that flew through a thin vapor of mercury atoms. They discovered that, when an electron collided with a mercury atom, it could lose only a specific quantity (4.9 electron volts) of its kinetic energy before flying away.[4] This energy loss corresponds to decelerating the electron from a speed of about 1.3 million meters per second to zero.[5] A faster electron does not decelerate completely after a collision, but loses precisely the same amount of its kinetic energy. Slower electrons merely bounce off mercury atoms without losing any significant speed or kinetic energy.

| Part of a series of articles about |
| Quantum mechanics |
|---|
|
These experimental results proved to be consistent with the Bohr model for atoms that had been proposed the previous year by Niels Bohr. The Bohr model was a precursor of quantum mechanics and of the electron shell model of atoms. Its key feature was that an electron inside an atom occupies one of the atom's "quantum energy levels". Before the collision, an electron inside the mercury atom occupies its lowest available energy level. After the collision, the electron inside occupies a higher energy level with 4.9 electron volts (eV) more energy. This means that the electron is more loosely bound to the mercury atom. There were no intermediate levels or possibilities in Bohr's quantum model. This feature was "revolutionary" because it was inconsistent with the expectation that an electron could be bound to an atom's nucleus by any amount of energy.[4][6]
In a second paper presented in May 1914, Franck and Hertz reported on the light emission by the mercury atoms that had absorbed energy from collisions.[7] They showed that the wavelength of this ultraviolet light corresponded exactly to the 4.9 eV of energy that the flying electron had lost. The relationship of energy and wavelength had also been predicted by Bohr because he had followed the structure laid out by Hendrik Lorentz at the 1911 Solvay Congress. At Solvay, Hendrik Lorentz suggested after Einstein’s talk on quantum structure that the energy of a rotator be set equal to nhv.[8][9] Therefore, Bohr had followed the instructions given in 1911 and copied the formula proposed by Lorentz and others into his 1913 atomic model.[10] Lorentz had been correct. The quantization of the atoms matched his formula incorporated into the Bohr model.[4] After a presentation of these results by Franck a few years later, Albert Einstein is said to have remarked, "It's so lovely it makes you cry."[1]
On December 10, 1926, Franck and Hertz were awarded the 1925 Nobel Prize in Physics "for their discovery of the laws governing the impact of an electron upon an atom".[11]
Experiment
Franck and Hertz's original experiment used a heated vacuum tube containing a drop of mercury; they reported a tube temperature of 115 °C, at which the vapor pressure of mercury is about 100 pascals (and far below atmospheric pressure).[2][12] A contemporary Franck–Hertz tube is shown in the photograph. It is fitted with three electrodes: an electron-emitting, hot cathode; a metal mesh grid; and an anode. The grid's voltage is positive relative to the cathode, so that electrons emitted from the hot cathode are drawn to it. The electric current measured in the experiment is due to electrons that pass through the grid and reach the anode. The anode's electric potential is slightly negative relative to the grid, so that electrons that reach the anode have at least a corresponding amount of kinetic energy after passing the grid.[13]
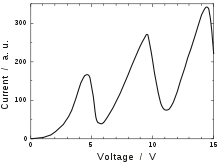
The graphs published by Franck and Hertz (see figure) show the dependence of the electric current flowing out of the anode upon the electric potential between the grid and the cathode.
- At low potential differences—up to 4.9 volts—the current through the tube increased steadily with increasing potential difference. This behavior is typical of true vacuum tubes that don't contain mercury vapor; larger voltages lead to larger "space-charge limited current".
- At 4.9 volts the current drops sharply, almost back to zero.
- The current then increases steadily once again as the voltage is increased further, until 9.8 volts is reached (exactly 4.9+4.9 volts).
- At 9.8 volts a similar sharp drop is observed.
- While it isn't evident in the original measurements of the figure, this series of dips in current at approximately 4.9 volt increments continues to potentials of at least 70 volts.[14]
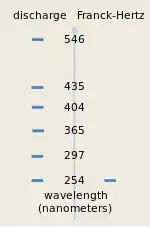
Franck and Hertz noted in their first paper that the 4.9 eV characteristic energy of their experiment corresponded well to one of the wavelengths of light emitted by mercury atoms in gas discharges. They were using a quantum relationship between the energy of excitation and the corresponding wavelength of light, which they broadly attributed to Johannes Stark and to Arnold Sommerfeld; it predicts that 4.9 eV corresponds to light with a 254 nm wavelength.[2] The same relationship was also incorporated in Einstein's 1905 photon theory of the photoelectric effect.[15] In a second paper, Franck and Hertz reported the optical emission from their tubes, which emitted light with a single prominent wavelength 254 nm.[7] The figure at the right shows the spectrum of a Franck–Hertz tube; nearly all of the light emitted has a single wavelength. For reference, the figure also shows the spectrum for a mercury gas discharge light, which emits light at several wavelengths besides 254 nm. The figure is based on the original spectra published by Franck and Hertz in 1914. The fact that the Franck–Hertz tube emitted just the single wavelength, corresponding nearly exactly to the voltage period they had measured, was very important.[13]
Modeling of electron collisions with atoms
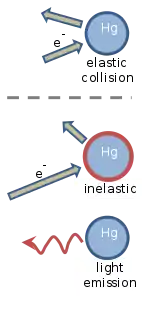
Franck and Hertz explained their experiment in terms of elastic and inelastic collisions between the electrons and the mercury atoms.[2][3] Slowly moving electrons collide elastically with the mercury atoms. This means that the direction in which the electron is moving is altered by the collision, but its speed is unchanged. An elastic collision is illustrated in the figure, where the length of the arrow indicates the electron's speed. The mercury atom is unaffected by the collision, mostly because it is about four hundred thousand times more massive than an electron.[16][17]
When the speed of the electron exceeds about 1.3 million meters per second,[5] collisions with a mercury atom become inelastic. This speed corresponds to a kinetic energy of 4.9 eV, which is deposited into the mercury atom. As shown in the figure, the electron's speed is reduced, and the mercury atom becomes "excited". A short time later, the 4.9 eV of energy that was deposited into the mercury atom is released as ultraviolet light that has a wavelength of precisely 254 nm. Following light emission, the mercury atom returns to its original, unexcited state.[16][17]
If electrons emitted from the cathode flew freely until they arrived at the grid, they would acquire a kinetic energy that's proportional to the voltage applied to the grid. 1 eV of kinetic energy corresponds to a potential difference of 1 volt between the grid and the cathode.[18] Elastic collisions with the mercury atoms increase the time it takes for an electron to arrive at the grid, but the average kinetic energy of electrons arriving there isn't much affected.[17]
When the grid voltage reaches 4.9 V, electron collisions near the grid become inelastic, and the electrons are greatly slowed. The kinetic energy of a typical electron arriving at the grid is reduced so much that it cannot travel further to reach the anode, whose voltage is set to slightly repel electrons. The current of electrons reaching the anode falls, as seen in the graph. Further increases in the grid voltage restore enough energy to the electrons that suffered inelastic collisions that they can again reach the anode. The current rises again as the grid potential rises beyond 4.9 V. At 9.8 V, the situation changes again. Electrons that have traveled roughly halfway from the cathode to the grid have already acquired enough energy to suffer a first inelastic collision. As they continue slowly towards the grid from the midway point, their kinetic energy builds up again, but as they reach the grid they can suffer a second inelastic collision. Once again, the current to the anode drops. At intervals of 4.9 volts this process will repeat; each time the electrons will undergo one additional inelastic collision.[16][17]
Early quantum theory
While Franck and Hertz were unaware of it when they published their experiments in 1914,[19] in 1913 Niels Bohr had published a model for atoms that was very successful in accounting for the optical properties of atomic hydrogen. These were usually observed in gas discharges, which emitted light at a series of wavelengths. Ordinary light sources like incandescent light bulbs emit light at all wavelengths. Bohr had calculated the wavelengths emitted by hydrogen very accurately.[20]
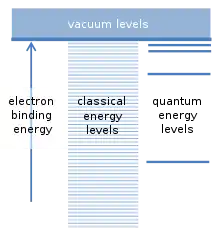
The fundamental assumption of the Bohr model concerns the possible binding energies of an electron to the nucleus of an atom. The atom can be ionized if a collision with another particle supplies at least this binding energy. This frees the electron from the atom, and leaves a positively charged ion behind. There is an analogy with satellites orbiting the earth. Every satellite has its own orbit, and practically any orbital distance, and any satellite binding energy, is possible. Since an electron is attracted to the positive charge of the atomic nucleus by a similar force, so-called "classical" calculations suggest that any binding energy should also be possible for electrons. However, Bohr assumed that only a specific series of binding energies occur, which correspond to the "quantum energy levels" for the electron. An electron is normally found in the lowest energy level, with the largest binding energy. Additional levels lie higher, with smaller binding energies. Intermediate binding energies lying between these levels are not permitted. This was a revolutionary assumption.[6]
Franck and Hertz had proposed that the 4.9 V characteristic of their experiments was due to ionization of mercury atoms by collisions with the flying electrons emitted at the cathode. In 1915 Bohr published a paper noting that the measurements of Franck and Hertz were more consistent with the assumption of quantum levels in his own model for atoms.[21] In the Bohr model, the collision excited an internal electron within the atom from its lowest level to the first quantum level above it. The Bohr model also predicted that light would be emitted as the internal electron returned from its excited quantum level to the lowest one; its wavelength corresponded to the energy difference of the atom's internal levels, which has been called the Bohr relation.[4] Franck and Hertz's observation of emission from their tube at 254 nm was also consistent with Bohr's perspective. Writing following the end of World War I in 1918, Franck and Hertz had largely adopted the Bohr perspective for interpreting their experiment, which has become one of the experimental pillars of quantum mechanics.[1][3] As Abraham Pais described it, "Now the beauty of Franck and Hertz's work lies not only in the measurement of the energy loss E2-E1 of the impinging electron, but they also observed that, when the energy of that electron exceeds 4.9 eV, mercury begins to emit ultraviolet light of a definite frequency ν as defined in the above formula. Thereby they gave (unwittingly at first) the first direct experimental proof of the Bohr relation!"[4] Franck himself emphasized the importance of the ultraviolet emission experiment in an epilogue to the 1960 Physical Science Study Committee (PSSC) film about the Franck–Hertz experiment.[19]
Experiment with neon

In instructional laboratories, the Franck–Hertz experiment is often done using neon gas, which shows the onset of inelastic collisions with a visible orange glow in the vacuum tube, and which also is non-toxic, should the tube be broken. With mercury tubes, the model for elastic and inelastic collisions predicts that there should be narrow bands between the anode and the grid where the mercury emits light, but the light is ultraviolet and invisible. With neon, the Franck–Hertz voltage interval is 18.7 volts, and an orange glow appears near the grid when 18.7 volts is applied. This glow will move closer to the cathode with increasing accelerating potential, and indicates the locations where electrons have acquired the 18.7 eV required to excite a neon atom. At 37.4 volts two distinct glows will be visible: one midway between the cathode and grid, and one right at the accelerating grid. Higher potentials, spaced at 18.7 volt intervals, will result in additional glowing regions in the tube.
An additional advantage of neon for instructional laboratories is that the tube can be used at room temperature. However, the wavelength of the visible emission is much longer than predicted by the Bohr relation and the 18.7 V interval. A partial explanation for the orange light involves two atomic levels lying 16.6 eV and 18.7 eV above the lowest level. Electrons excited to the 18.7 eV level fall to the 16.6 eV level, with concomitant orange light emission.[22]
References
- Rice, Stuart A.; Jortner, Joshua (2010). "James Franck 1882-1964: A Biographical Memoir" (PDF). National Academy of Sciences (US). p. 6.
Our understanding of the world was transformed by the results of this experiment; it is arguably one of the most important foundations of the experimental verification of the quantum nature of matter.
- Franck, J.; Hertz, G. (1914). "Über Zusammenstöße zwischen Elektronen und Molekülen des Quecksilberdampfes und die Ionisierungsspannung desselben" [On the collisions between electrons and molecules of mercury vapor and the ionization potential of the same] (PDF). Verhandlungen der Deutschen Physikalischen Gesellschaft (in German). 16: 457–467. A translation of this paper is given in Boorse, Henry A.; Motz, Lloyd (1966). "46. The Quantum Theory is Tested". The World of the Atom. Vol. 1. Basic Books. pp. 766–778. OCLC 534667. In their initial papers, Franck and Hertz interpreted the 4.9 V potential associated with inelastic electron-mercury collisions as indicative of the ionization potential of mercury. The relationship to the Bohr model of atoms emerged somewhat later.
- Lemmerich, Jost (2011). Science and Conscience: The Life of James Franck. Translated by Ann Hentschel. Stanford University Press. pp. 45–50. ISBN 9780804779098.
Then two papers by Franck and Hertz about measurements on vaporized mercury that were to enter their names on the rolls of the history of physics appeared in quick succession. The first paper was presented by Gustav Hertz at the German Physical Society's meeting on 24 April 1914, the second by James Franck on May 22. (p. 45)
Translation of Aufrecht im Sturm der Zeit : der Physiker James Franck, 1882-1964. Verlag für Geschichte der Naturwissenschaften und der Technik. 2007. ISBN 9783928186834. OCLC 234125038. - Pais, Abraham (1995). "Introducing Atoms and Their Nuclei". In Brown, Laurie M.; Pais, Abraham; Pippard, Brian (eds.). Twentieth Century Physics. Vol. 1. American Institute of Physics Press. p. 89. ISBN 9780750303101.
Now the beauty of Franck and Hertz's work lies not only in the measurement of the energy loss E2-E1 of the impinging electron, but they also observed that, when the energy of that electron exceeds 4.9 eV, mercury begins to emit ultraviolet light of a definite frequency ν as defined in the above formula. Thereby they gave (unwittingly at first) the first direct experimental proof of the Bohr relation!
The frequency ν is related to the wavelength λ of light by the formula ν = c/λ, where c=2.99×108 meters per second is the speed of light in vacuum. - For converting electron volts to electron speeds, see "The speed of electrons". Practical Physics. Nuffield Foundation. Retrieved 2014-04-18.
- Cohen, I. Bernard (1985). Revolution in Science. Belknap Press. pp. 427–428. ISBN 9780674767775.
In 1912 a young Dane working in Rutherford's laboratory in Manchester proposed a revolutionary new model of the atom. ... What made Bohr's theory difficult to believe in was the idea of discrete and fixed states or orbits, with no intermediate states being possible.
- Franck, J.; Hertz, G. (1914). "Über die Erregung der Quecksilberresonanzlinie 253,6 μμ durch Elektronenstöße" [On the excitation of mercury resonance lines at 253.6 nm by electron collisions]. Verhandlungen der Deutschen Physikalischen Gesellschaft (in German). 16: 512–517. The symbol μμ is an outdated, rare usage for a nanometer. This article was reprinted in Franck, James; Hertz, Gustav; Hermann, Armin (1967). Die Elektronenstoßversuche. München: E. Battenberg. OCLC 9956175.
- Original Proceedings of the 1911 Solvay Conference published 1912. THÉORIE DU RAYONNEMENT ET LES QUANTA. RAPPORTS ET DISCUSSIONS DELA Réunion tenue à Bruxelles, du 30 octobre au 3 novembre 1911, Sous les Auspices dk M. E. SOLVAY. Publiés par MM. P. LANGEVIN et M. de BROGLIE. Translated from the French, p.447.
- Heilbron, John L., and Thomas S. Kuhn. “The Genesis of the Bohr Atom.” Historical Studies in the Physical Sciences, vol. 1, University of California Press, 1969, pp. vi–290, p. 244 https://doi.org/10.2307/27757291.
- See Bohr model
- Oseen, C. W. (December 10, 1926). "Nobel Prize in Physics 1925 - Presentation Speech". The Nobel Foundation.
- Huber, Marcia L.; Laesecke, Arno; Friend, Daniel G. (April 2006). "The vapor pressure of mercury" (PDF). National Institute of Standards. p. 5. NISTIR 6643.
- Brandt, Siegmund (2008). "25. The Franck Hertz experiment (1914)". The harvest of a century : discoveries of modern physics in 100 episodes. Oxford University Press. p. 272. ISBN 9780191580123.
- Thornton, Stephen; Rex, Andrew (2012). Modern Physics for Scientists and Engineers (4 ed.). Cengage Learning. pp. 154–156. ISBN 9781133103721.
- Pais, Abraham (1982). Subtle is the Lord: The Science and the Life of Albert Einstein. Oxford University Press. p. 381. ISBN 9780191524028. The energy E of a photon is the product of Planck's constant h and the ratio c/λ of the speed of light c and the wavelength λ.
- Melissinos, Adrian Constantin; Napolitano, Jim (2003). "1.3 The Franck–Hertz Experiment". Experiments in Modern Physics. Gulf Professional Publishing. pp. 10–19. ISBN 9780124898516. This reference incorrectly suggests that Franck and Hertz were aware of the Bohr model when they published their experiments. Franck himself remarked on this in an interview late in his life; see Holton, Gerald (1961). "On the recent past of physics". American Journal of Physics. 61 (12): 805–810. Bibcode:1961AmJPh..29..805H. doi:10.1119/1.1937623.
- Demtröder, Wolfgang (2010). "3.4.4 Franck–Hertz experiment". Atoms, Molecules and Photons: An Introduction to Atomic-, Molecular- and Quantum Physics. Springer. pp. 118–120. ISBN 9783642102981.
- In their original experiment, Franck and Hertz used platinum for both the cathode and the grid. When different materials are used for the electrodes, there is an additional contribution to the kinetic energy beyond the externally applied voltage. See Thornton, Stephen; Rex, Andrew (2012). Modern Physics for Scientists and Engineers (4 ed.). Cengage Learning. pp. 154–156. ISBN 9781133103721.
- In 1960, Franck explained that he and Hertz were unaware of Bohr's ideas when their two 1914 papers were presented. Franck gave his remarks as the epilogue to the film on the Franck–Hertz experiment from the Physical Science Study Committee (1960). The film is available online; see Byron L. Youtz (narrator); James Franck (epilogue); Jack Churchill (director) (1960). Franck-Hertz experiment (16 mm film). Educational Services. 25 minutes in. OCLC 4949442. Retrieved 2014-07-01.. A transcript of the epilogue was published shortly after the film was made; see Holton, Gerald (1961). "On the recent past of physics". American Journal of Physics. 61 (12): 805–810. Bibcode:1961AmJPh..29..805H. doi:10.1119/1.1937623.
- Heilbron, John L. (1985). "Bohr's First Theories of the Atom". In French, A. P.; Kennedy, P. J. (eds.). Niels Bohr: A Centenary Volume. Cambridge, Massachusetts: Harvard University Press. pp. 33–49. ISBN 9780674624160. OCLC 12051112.
- Kragh, Helge (2012). Niels Bohr and the Quantum Atom: The Bohr Model of Atomic Structure 1913-1925. Oxford University Press. p. 144. ISBN 9780191630460. Kragh quotes a sentence from one of Bohr's 1915 papers in which he discusses the 1914 papers by Franck and Hertz: "It seems that their experiment may possibly be consistent with the assumption that this voltage (4.9 V) corresponds only to the transition from the normal state to some other stationary state of the neutral atom."
- Csele, Mark (2011). "2.6 The Franck–Hertz Experiment". Fundamentals of Light Sources and Lasers. John Wiley & Sons. pp. 31–36. ISBN 9780471675228.
Further reading
- Basile, Giorgio. "3B Scientific Mercury Franck–Hertz Tube U8482170". Selection of images of a vacuum tube used for the Franck–Hertz experiment in instructional laboratories.
- Franck, James (1965). "Transformation of Kinetic Energy of Free Electrons into Excitation Energy of Atoms by Impacts" (PDF). Nobel Lectures, Physics 1922–1941. Elsevier. Translation of Franck's Nobel lecture that he gave December 11, 1926.
- Gearhart, Clayton A. (2014). "The Franck-Hertz Experiments, 1911–1914: Experimentalists in Search of a Theory". Physics in Perspective. 16 (3): 293–343. Bibcode:2014PhP....16..293G. doi:10.1007/s00016-014-0139-3. S2CID 118257144.
- Hertz, Gustav (1965). "The results of the electron-impact tests in the light of Bohr's theory of atoms" (PDF). Nobel Lectures, Physics 1922–1941. Elsevier. Translation of Hertz's Nobel lecture that he gave December 11, 1926.
- Nicoletopoulos, Peter (2012). "Up-to-date literature on the Franck–Hertz Experiment". Archived from the original on 2012-01-16. See also "Up-to-date literature on the Franck–Hertz experiment". Nicoletopoulos, who died in 2013, had authored and co-authored several papers related to the Franck–Hertz experiment; these papers challenge the conventional interpretations of the experiment. See Robson, Robert; White, Ronald. "In Memory of Peter Nicoletopoulos" (PDF). ARC Centre of Excellence for Antimatter–Matter Studies: Annual Report 2012. Australian Research Council. p. 3. Archived from the original (PDF) on 2014-01-24. Retrieved 2014-03-29.
- Rapior, G.; Sengstock, K.; Baev, V. (2006). "New features of the Franck–Hertz experiment" (PDF). Am. J. Phys. 74 (5): 423–428. Bibcode:2006AmJPh..74..423R. doi:10.1119/1.2174033. Archived from the original (PDF) on 2014-04-13. Retrieved 2014-03-30. Franck and Hertz's original paper reported anode currents up to about 15 V, as illustrated in the figure above. Additional maxima and minima occur when current is measured to higher voltages. This paper notes that the spacing between the minima and maxima isn't exactly 4.9 V, but increases for higher voltages and varies with temperature, and provides a model for this effect.