Cultured meat
Cultured meat (also known by other names) is meat produced by culturing animal cells in vitro.[1] It is a form of cellular agriculture.[2]

Cultured meat is produced using tissue engineering techniques pioneered in regenerative medicine.[3] Jason Matheny popularized the concept in the early 2000s after he co-authored a paper[4] on cultured meat production and created New Harvest, the world's first nonprofit organization dedicated to in-vitro meat research.[5]
Cultured meat has the potential to address the environmental impact of meat production, animal welfare, food security and human health.[6][7][8][9][10] in addition to its potential mitigation of climate change.[2]
In 2013, Mark Post created the first hamburger patty grown directly from cells. Since then, other cultured meat prototypes have gained media attention: SuperMeat opened a farm-to-fork restaurant called "The Chicken"[11] in Tel Aviv to test consumer reaction to its "Chicken" burger,[12] while the "world's first commercial sale of cell-cultured meat" occurred in December 2020 at Singapore restaurant 1880, where cultured meat manufactured by US firm Eat Just was sold.[13]
While most efforts focus on common meats such as pork, beef, and chicken which comprise the bulk of consumption in developed countries,[14] companies such as Orbillion Bio focused on high end or unusual meats including elk, lamb, bison, and Wagyu beef.[15] Avant Meats brought cultured grouper to market,[16] while other companies pursued additional fish species and other seafood.[17]
The production process is constantly evolving, driven by companies and research institutions.[18] The applications for cultured meat led to ethical, health, environmental, cultural, and economic discussions.[19] Data published by the non-governmental organization Good Food Institute found that in 2021 cultivated meat companies attracted $140 million in Europe.[2] Cultured meat is served at special events and a few high end restaurants. Mass production has not yet started.
Nomenclature
Besides cultured meat, the terms healthy meat,[20] slaughter-free meat,[21] in vitro meat, vat-grown meat,[22] lab-grown meat,[23] cell-based meat,[24] clean meat,[25] cultivated meat[26] and synthetic meat[27] have been used to describe the product. Artificial meat is occasionally used,[28] although that specific term has multiple definitions.
Between 2016 and 2019, clean meat gained traction. The Good Food Institute (GFI) coined the term in 2016,[29] and in late 2018, the institute published research claiming that use of clean better reflected the production process and benefits.[30][31] By 2018 it had surpassed cultured and "in vitro" in media mentions and Google searches.[32] Some industry stakeholders felt that the term unnecessarily tarnished conventional meat producers, continuing to prefer cell-based meat as a neutral alternative.[33][34]
In September 2019, GFI announced new research which found that the term cultivated meat is sufficiently descriptive and differentiating, possesses a high degree of neutrality, and ranks highly for consumer appeal.[26][35] A September 2021 poll indicated that the majority of industry CEOs have a preference for cultivated meat, with 75 percent of 44 companies preferring it.[36]
History
Initial research
The theoretical possibility of growing meat in an industrial setting has long been of interest. In a 1931 essay published by various periodicals and later included in his work Thoughts and Adventures, British statesman Winston Churchill wrote: "We shall escape the absurdity of growing a whole chicken to eat the breast or wing, by growing these parts separately under a suitable medium."[37]
In the 1950s, Dutch researcher Willem van Eelen independently came up with the idea for cultured meat. As a prisoner of war during the Second World War, Van Eelen suffered from starvation, leaving him passionate about food production and food security.[38] He attended a university lecture discussing the prospects of preserved meat.[39] The earlier discovery of cell lines provided the basis for the idea.
In vitro cultivation of muscle fibers was first performed successfully in 1971 when pathologist Russel Ross cultured guinea-pig aorta.
In 1991, Jon F. Vein secured patent US 6835390 for the production of tissue-engineered meat for human consumption, wherein muscle and fat would be grown in an integrated fashion to create food products.[40]
In 2001, dermatologist Wiete Westerhof along with van Eelen and businessperson Willem van Kooten announced that they had filed for a worldwide patent on a process to produce cultured meat.[41] The process empoyed a matrix of collagen seeded with muscle cells bathed in a nutritious solution and induced to divide.[42]
That same year, NASA began conducting cultured meat experiments, with the intent of allowing astronauts to grow meat instead of transporting it. In partnership with Morris Benjaminson, they cultivated goldfish and turkey.[43]
In 2003, Oron Catts and Ionat Zurr exhibited a few centimeters of "steak", grown from frog stem cells, which they cooked and ate. The goal was to start a conversation surrounding the ethics of cultured meat—"was it ever alive?", "was it ever killed?", "is it in any way disrespectful to an animal to throw it away?"[44]
In the early 2000s, American public health student Jason Matheny traveled to India and visited a factory chicken farm. He was appalled by the implications of this system. Matheny later teamed up with three scientists involved in NASA's efforts. In 2004, Matheny founded New Harvest to encourage development by funding research.In 2005 the four published the first peer-reviewed literature on the subject. [45]
In 2008, PETA offered a $1 million prize to the first company to bring cultured chicken meat to consumers by 2012.[46] The contestant was required to complete two tasks to earn the prize:
- produce a cultured chicken meat product that was indistinguishable from real chicken and
- produce the product in large enough quantities to be competitively sold in at least 10 states.
The contest was later extended until 4 March 2014. The deadline eventually expired without a winner.[47]
In 2008, the Dutch government invested $4 million into experiments regarding cultured meat.[48] The In Vitro Meat Consortium, a group formed by international researchers, held the first international conference hosted by the Food Research Institute of Norway in April.[49] Time magazine declared cultured meat production to be one of the 50 breakthrough ideas of 2009.[50] In November 2009, scientists from the Netherlands announced they had managed to grow meat using cells from a live pig.[51]
First public trial

The first cultured beef burger patty was created by Mark Post at Maastricht University in 2013.[52] It was made from over 20,000 thin strands of muscle tissue, cost over $300,000 and needed 2 years to produce.[53]
The burger was tested on live television in London on 5 August 2013. It was cooked by chef Richard McGeown of Couch's Great House Restaurant, Polperro, Cornwall, and tasted by critics Hanni Rützler, a food researcher from the Future Food Studio, and Josh Schonwald. Rützler stated, "There is really a bite to it, there is quite some flavour with the browning. I know there is no fat in it so I didn't really know how juicy it would be, but there is quite some intense taste; it's close to meat, it's not that juicy, but the consistency is perfect. This is meat to me... It's really something to bite on and I think the look is quite similar." Rützler added that even in a blind trial she would have taken the product for meat rather than a soya copy.[54]
Industry development

It's just a matter of time before this is gonna happen, I'm absolutely convinced of that. In our case, I estimate the time to be about 3 years before we are ready to enter the market on a small scale, about 5 years to enter the market on a larger scale, and if you'd ask me: "When will [cultured meat] be in the supermarket around the corner?" That'll be closer to 10 than to 5 years, I think.
Peter Verstrate, Mosa Meat (2018)[55]: 1:06:15
Between 2011 and 2017, many cultured meat startups were launched. Memphis Meats (now Upside Foods[56]) launched a video in February 2016, showcasing its cultured beef meatball.[57][58][59] In March 2017, it showcased chicken tenders and duck a l'orange, the first cultured poultry shown to the public.[60][61][62]
An Israeli company, SuperMeat, ran a crowdfunding campaign in 2016, for its work on cultured chicken.[63][64][65][66][67]
Finless Foods, a San Francisco-based company working on cultured fish, was founded in June 2016. In March 2017 it commenced laboratory operations.[68]
In March 2018, Eat Just (in 2011 founded as Hampton Creek in San Francisco, later known as Just, Inc.) claimed to be able to offer a consumer product from cultured meat by the end of 2018. According to CEO Josh Tetrick the technology was already there. JUST had about 130 employees and a research department of 55 scientists, where cultured meat from poultry, pork and beef was researched. JUST has received investments from Chinese billionaire Li Ka-shing, Yahoo! co-founder Jerry Yang and according to Tetrick also by Heineken International and others.[69]
There is a handful [of startups]. It's quite interesting to see, there are three hubs: one in Silicon Valley, one in the Netherlands and one in Israel. I think that's because these three places have firstly, a great agricultural university – we've got Wageningen; secondly, a great medical university – for us that's Leiden; and finally we've got Delft on the engineering side. Those three combined gives you a firm basis to [develop cultured meat], and that [combination] exists in Israel, the Netherlands and America.
Krijn de Nood, Meatable (2020)[70]
Dutch startup Meatable, consisting of Krijn de Nood, Daan Luining, Ruud Out, Roger Pederson, Mark Kotter and Gordana Apic among others, reported in September 2018 that it had succeeded in growing meat using pluripotent stem cells from animal umbilical cords. Although such cells are reportedly difficult to work with, Meatable claimed to be able to direct them to behave to become muscle or fat cells as needed. The major advantage is that this technique bypasses fetal bovine serum, meaning that no animal has to be killed to produce meat.[71] That month, an estimated 30 cultured meat startups operated across the world.[55]
Integriculture is a Japan-based company working on their CulNet system. Competitors included England based Multus Media and Canadian Future Fields.[72]
In August 2019, five American startups announced the formation of the Alliance for Meat, Poultry & Seafood Innovation (AMPS Innovation), a coalition seeking to work with regulators to create a pathway to market for cultured meat and seafood.[73] The founding members include Eat Just, Memphis Meats, Finless Foods, BlueNalu, and Fork & Goode.[74] Similarly in December 2021, a group of 13 European and Israeli companies (Aleph Farms, Bluu Biosciences, Cubiq Foods, Future Meat, Gourmey, Higher Steaks, Ivy Farm, Meatable, Mirai Foods, Mosa Meat, Peace of Meat, SuperMeat, and Vital Meat) established Cellular Agriculture Europe, a Belgium-based association that sought to 'find common ground and speak with a shared voice for the good of the industry, consumers, and regulators'.[75][76][77]
In 2019, the Foieture project was launched in Belgium with the goal of developing cultured foie gras (the name is a portmanteau of 'foie' and 'future') by a consortium of 3 companies (cultured-meat startup Peace of Meat, small meat-seasoning company Solina, and small pâté-producing company Nauta) and 3 non-profit institutes (university KU Leuven, food industry innovation centre Flanders Food, and Bio Base Europe Pilot Plant).[78] Peace of Meat stated in December 2019 that it intended to complete its proof of concept in 2020, to produce its first prototype in 2022, and to go to market in 2023.[78] That month, the Foieture project received a research grant of almost 3.6 million euros from the Innovation and Enterprise Agency of the Flemish Government.[78] In May 2020, Peace of Meat's Austrian-born cofounder and scientific researcher Eva Sommer stated that the startup was then able to produce 20 grams of cultured fat at a cost of about 300 euros (€15,000/kg); the goal was to reduce the price to 6 euros per kilogram by 2030.[79] Piece of Meat built two laboratories in the Port of Antwerp.[79] In late 2020, MeaTech acquired Peace of Meat for 15 million euros, and announced in May 2021 that it would build a new large-scale pilot plant in Antwerp by 2022.[80]
In 2019, Aleph Farms collaborated with 3D Bioprinting Solutions to culture meat on the International Space Station. This was done by extruding meat cells onto a scaffold using a 3D printer.[81]
In January 2020, Quartz found around 30 cultured meat startups, and that Memphis Meats, Just Inc. and Future Meat Technologies were the most advanced because they were building pilot plants.[82][83] According to New Scientist in May 2020, 60 start-ups were developing cultured meat. Some of these were technology suppliers.[84] Growth media reportedly still cost "hundreds of dollars per litre, but for clean meat production to scale this needs to drop to around $1 a litre."[84] In June 2020, Chinese government officials called for a national strategy to compete in cultured meat.[85]
In November 2020, Indian start-up Clear Meat claimed it had managed to cultivate chicken mince at the cost of only 800–850 Indian rupees (US$10.77–11.44), while a slaughtered processed chicken cost about 1000 rupees.[86]
Market entry
In the European Union, novel foods such as cultured meat products have to go through a testing period of about 18 months during which a company must prove to the European Food Safety Authority (EFSA) that their product is safe.[87]
In November 2020, SuperMeat opened a 'test restaurant' in Ness Ziona, Israel, right next to its pilot plant; journalists, experts and a small number of consumers could book an appointment to taste the novel food there, while looking through a glass window into the production facility on the other side. The restaurant was not yet fully open to the public, because as of June 2021 SuperMeat still needed to wait for regulatory approval to start mass production for public consumption, and because the Covid-19 pandemic restricted restaurant operations.[88][89]
On 2 December 2020, the Singapore Food Agency approved the "chicken bites" produced by Eat Just for commercial sale. It marked the first time that a cultured meat product passed the safety review (which took 2 years) of a food regulator, and was widely regarded as a milestone for the industry. The chicken bits were scheduled for introduction in Singaporean restaurants.[90] Restaurant "1880" became the first to serve cultured meat to customers on Saturday 19 December 2020.[91][92]
In March 2022, cultured meat producers had reached the level of attempting to gain regulatory approval from European Union supranational institutions coming just before mass goods could be sold to consumers.[2]
On April 27, 2022, the European Commission approved the request for the collection of signatures for the European Citizens' Initiative End The Slaughter Age to shift subsidies from animal husbandry to cellular agriculture.[93]
Companies
Note: dates in italics refer to projected dates of achievement in the future; they may shift.
| Name | Founded | Area | Focus | Recent costs | Proof of concept | Pilot plant | Market entry |
|---|---|---|---|---|---|---|---|
| Aleph Farms | 2017[94] | Beef | Over $3,000/kg (Nov 2019 claim)[95] | Dec 2018[94] | Feb 2022[96] | End 2022 (Feb 2022 claim)[96] | |
| Ants Innovate | 2020 | Pork | |||||
| Appleton Meats | 2016 | Beef | |||||
| Artemys Foods | 2019 | Meat | Fall 2020[97] | ||||
| Avant Meats | 2018[98] | Fish protein | November 2019[99] | 2022 (Aug 2020 claim)[98] | |||
| Balletic Foods[100] | |||||||
| Because Animals[101] | 2018 | Pet food | May 2019[102] | 2022 (Aug 2021 claim)[103] | |||
| Biftek[104] | 2018[105] | Culture media | |||||
| BioBQ | 2018 | Scaffolding | 2022[106] | ||||
| BlueNalu | 2018 | Seafood | Fall 2019[107] | ||||
| BioTech Foods (acquired by JBS[108]) |
2017[87] | Pork[87] | €100/kg (July 2019 claim)[109] | 2020[110] | mid-2024 (Dec 2021 claim)[108] | ||
| Cell Ag Tech | 2018 | Meat | |||||
| Cell Farm Food Tech | 2018 | Meat | |||||
| CellX | 2020[111] | Pork | 2021[112][113] | (by 2025) aiming for cost-parity with conventionally sourced pork[114] | |||
| Clear Meat[115] | 2019[115] | Poultry[115] | c. 825 rupees/chicken (Nov 2020 claim)[86] | 2022 (May 2019 claim)[116] | |||
| Cubiq Foods | 2018 | Fat | Sep 2019[117] | ||||
| Cultured Food Innovation Hub[118] | 2021[118] | Meat[118] | 2022 (Sept 2021 claim)[118] | ||||
| Eat Just | 2011 | Meat | C. €50/nugget (Jan 2020 claim)[119] | Dec 2017[120] | Constructing (Jan 2020)[82] | December 2020 (restaurants)[90] | |
| Finless Foods | 2016[121] | Tuna | $7,000/lb (Feb 2018 claim)[122] | Sep 2017[122] | Constructing (Oct 2021)[123] | 2022[123] | |
| Fork & Goode | 2018 | Meat | |||||
| Future Fields | 2017 | Culture media | |||||
| Future Meat Technologies | 2018 | Meat | $10/lb (Feb 2020 goal by 2022)[124] | 2019 | June 2021[125] | 2022 (Oct 2019 claim)[126] | |
| Gaia Foods | 2019 | Red meat | |||||
| Gourmey | 2019 | Foie gras | |||||
| Heuros | 2017 | Pet food | |||||
| Higher Steaks | 2017 | Pork | £'Thousands'/kg (July 2020 claim)[127] | July 2020[128] | |||
| Hoxton Farms | 2020 | Fat | |||||
| IntegriCulture, Inc. | 2015 | Foie gras | ¥20,000/kg (July 2019 claim)[129] | 2021[130] | 2021 (July 2020 claim) | ||
| Matrix Meats | 2019 | Scaffolding | 2020[131] | ||||
| Meatable | 2018 | Pork | End 2020[132] | Preparing (Sept 2021)[133][134] | 2023 (Apr 2021 claim)[132] | ||
| Meatleo | 2021 | Beef | |||||
| MeaTech (subsidiary: Peace of Meat) |
2019 | Foie gras | €15,000/kg (May 2020 claim)[79] | 4 March 2020[135] | Constructing; 2022 (May 2021 claim)[80] | 2023 (Dec 2019 claim)[78] | |
| Mewery | 2020 | Pork | mid 2022 | 2025 | |||
| Mirai Foods | 2020 | Beef | 'Small car'/kg (June 2020 claim)[136] | June 2020[136] | |||
| Mosa Meat / Maastricht University |
2015 | Beef | €60/kg (Feb 2017 goal by 2020)[137] '88x cheaper' (July 2020 claim)[138] |
Aug 2013 (UM)[54] | Installing (May 2020)[138] | 2022 (Feb 2020 claim)[139] | |
| Motif FoodWorks | 2019[140] | Beef | End 2020 (Aug 2020 claim)[141] | Q4 2021 (beef flavouring) (Oct 2020 claim)[142] | |||
| Multus Media | 2019 | Culture media | October 2019[143] | ||||
| New Age Meats | 2018[144] | Pork | Sep 2018[145] | Constructing (Oct 2021)[146] | 2022[146] | ||
| SavorEat | 2016[94] | Beef | Mid-2021 (restaurants) (May 2020 claim)[94] | ||||
| Shiok Meats | 2018[147] | Shrimp | $3,500/kg (Oct 2020 claim)[148] | 2019[148] | 2021 (March 2020 claim)[149][147][150] | ||
| Shojinmeat Project[151] | |||||||
| SuperMeat | 2015[94] | Poultry | $35/burger (Dec 2020 claim)[88] | 2018[152] | November 2020[89] | By 2022 (May 2020 claim)[94] Test restaurant Nov 2020[89] | |
| Upside Foods (formerly Memphis Meats) |
2015 | Poultry | $1,700/lb (Feb 2018 claim)[153] | Feb 2016[154] | 4 November 2021[155][156] | Around 2020 (Feb 2017 claim)[137] | |
| Vow | 2019[157] | Kangaroo | US$1350/kg (Aug 2019 claim)[158] | Aug 2019[158] | Oct 2022[159] | 2022 (restaurants) (Oct 2019 claim)[160] | |
| Wildtype Foods | 2016 | Salmon | June 2019[161] | 24 June 2021[162] |
Aside from these companies, non-profit organisations such as New Harvest, the Good Food Institute, ProVeg International[163] and the Cellular Agriculture Society advocate for, fund and research cultured meat.[164] Impossible Foods markets the Impossible Burger, which contains heme that the company claims gives the burger its bloody look and taste. Their soy leghemoglobin is produced by taking the soybean gene that encodes the heme protein and transferring it to yeast.[165]
Pilot plants
Note: data in italics refer to unfinished projects or projected capacities in the future; they may shift.
| Company | Location | In service | Capacity |
|---|---|---|---|
| Aleph Farms | Rehovot, Israel[166] | Feb 2022[96] | (3,000 m2[166]). Fully operational by summer 2022[96] |
| BioTech Foods (acquired by JBS[108]) |
San Sebastián, Spain[108] | 2020[110] | |
| Eat Just | San Francisco, California[123] | Constructing (Jan 2020)[82] | (20+ 1200L bioreactors[123]) |
| Finless Foods | Emeryville, California[123] | Constructing (Oct 2021)[123] | |
| Future Meat Technologies | Rehovot, Israel[166] | June 2021[125] | 500 kilograms per day (182,625 kg/y)[166] |
| Meatable & DSM | Delft, Netherlands[134][133] | Preparing (Sept 2021)[134][133] | 5,000 kilograms per day by 2025[132] |
| MeaTech / Peace of Meat | Antwerp, Belgium[78] | 2 labs March 2020[135] | 700 grams per production run[167] |
| Antwerp, Belgium[80] | Constructing plant (May 2021)[80] | ||
| Mosa Meat | Maastricht, Netherlands[168] | Installing (May 2020)[138] | 100 kilograms per month (1,200 kg/y) per 200L bioreactor[169][168] (scalable to 180,000 kg/y)[168] |
| New Age Meats | Alameda, California[146] | Constructing (Oct 2021)[146] | (20,000 square feet)[146] |
| SuperMeat | Ness Ziona, Israel[88][89] | November 2020[88][89] | "Hundreds of kilograms" per week (June 2021)[89] |
| Upside Foods (Memphis Meats) |
Emeryville, California[155] | 4 November 2021[155] | 22,680 kilograms (50,000 pounds) per year[155][156] (scalable to 400,000 lbs/y / 181,440 kg/y)[156] |
| Wildtype Foods | San Francisco, California[123] | 24 June 2021[162] | 50,000 pounds (22,680 kg) salmon per year[123] (scalable to 200,000 lbs/y / 90,718 kg/y)[123] |
Process
Cell lines
Cellular agriculture requires cell lines, generally stem cells. Stem cells are undifferentiated cells which have the potential to become many or all of the required kinds of specialized cell types. Totipotent stem cells have the capacity to differentiate into all the different cell types found within the body. Pluripotent stem cells can mature into all cell types save those in the placenta, and multipotent stem cells can differentiate into several specialized cells within one lineage. Unipotent stem cells can differentiate into one specific cell fate.[170]
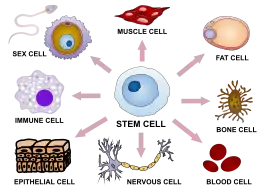
While pluripotent stem cells would be an ideal source, the most prominent example of this subcategory is embryonic stem cells which—due to ethical issues—are controversial for use in research. As a result, scientists have developed induced pluripotent stem cells (iPSCs)—essentially multipotent blood and skin cells that have been regressed to a pluripotent state enabling them to differentiate into a greater range of cells.[171] The alternative is using multipotent adult stem cells that give rise to muscle cell lineages or unipotent progenitors which differentiate into muscle cells.[170]
Favourable characteristics of stem cells include immortality, proliferative ability, unreliance on adherence, serum independence and easy differentiation into tissue. However, the natural presence of such characteristics are likely to differ across cell species and origin. As such, in vitro cultivation must be adjusted to fill the exact needs of a specific cell line. With regards to immortality, cells have a limit on the number of times they can divide that is dictated by their telomere cap—supplementary nucleotide bases added to the end of their chromosomes. With each division, the telomere cap progressively shortens until nothing remains, in which case the cell ceases to divide. By inducing pluripotency, the telomere cap can be lengthened such that the cell divides indefinitely.[171]
Cell lines can be collected from a primary source, i.e., through a biopsy on an animal under local anesthesia. They could also be established from secondary sources such as cryopreserved cultures (cultures frozen after previous research).
Growth medium
Once cell lines are established, they are immersed in a culture media to induce them to proliferate. Culture media are typically formulated from basal media that provide cells with necessary carbohydrates, fats, proteins and salts. Once a cell consumes a sufficient amount, it divides and the population increases exponentially. Culture media can be supplemented with additives—for instance sera—that supply additional growth factors. Growth factors can be secreted proteins or steroids that are crucial in regulating cellular processes.[1]
Once differentiation begins, muscle fibres begin to contract and generate lactic acid. Cells' ability to absorb nutrients and proliferate in part depends on the pH of their environment. As lactic acid accumulates within the media, the environment will become progressively more acidic and falls below the optimal pH. As a result, culture media must be frequently refreshed. This helps refresh the concentration of nutrients from the basal media.[18]
Scaffold
.jpg.webp)
In the case of structured meat products—products that are characterized by their overall configuration as well as cell type—cells must be seeded to scaffolds. Scaffolds are essentially molds meant to reflect and encourage the cells to organize into a larger structure. When cells develop in vivo, they are influenced by their interactions with the extracellular matrix (ECM). The ECM is the 3-dimensional mesh of glycoproteins, collagen and enzymes responsible for transmitting mechanical and biochemical cues to the cell. Scaffolds need to simulate the characteristics of the ECM.[1] Key properties:
Porosity
Pores are minute openings on the surface of the scaffold. They can be created on the surface of the biomaterial in order to release cellular components that could interfere with tissue development. They also help diffuse gas and nutrients to the innermost layers of adherent cells which prevents developing a "necrotic center" (created when cells that are not in direct contact with the culture medium die due to a lack of nutrients).[172]
Vascularization
Vascular tissue found in plants contains the organs responsible for internally transporting fluids. It forms natural topographies that provide a low cost way to promote cell alignment by replicating the natural physiological state of myoblasts. It may also help with gas and nutrient exchange.[172]
Biochemical properties
A scaffold's biochemical properties should be similar to those of the ECM. It must facilitate cell adhesion through textural qualities or chemical bonding. Additionally, it must produce the chemical cues that encourage cell differentiation. Alternatively, the material should be able to blend with other substances which have these functional qualities.[172]
Crystallinity
The degree of a material's crystallinity determines qualities such as rigidity. High crystallinity can be attributed to hydrogen bonding which in turn increases thermal stability, tensile strength (important for maintaining the scaffold's shape), water retention (important for hydrating the cells) and Young's modulus.[172]
Degradation
Certain materials degrade into compounds that are beneficial to cells, although this degradation can also be irrelevant or detrimental. Degradation allows easy removal of the scaffold from the finished product leaving only animal tissue—thereby increasing its resemblance to in vivo meat. This degradation can be induced by exposure to certain enzymes which do not impact the muscle tissue.[172]
Edibility
If scaffolds are unable to be removed from the animal tissue, they must be edible to ensure consumer safety. It would be beneficial if they were to be made out of nutritious ingredients.[172]
Since 2010, academic research groups and companies have emerged in order to identify raw materials that have the characteristics of suitable scaffolds.[172][173][174][175][176][177]
Cellulose
Cellulose is the most abundant polymer in nature and provides the exoskeletons of plant leaves. Due to its abundance, it can be obtained at a relatively low cost. It is also versatile and biocompatible. Through a process called "decellularization", it is coated in a surfactant that creates pores. These pores release the plant's cellular components, and it becomes decellularized plant tissue. This material has been extensively studied by the Pelling and Gaudette Groups at University of Ottawa and Worcester Polytechnic Institute, respectively. Through cross-linking (forming covalent bonds between individual polymer chains to hold them together) the plant tissue's mechanical properties can be changed so that it more closely resembles muscle tissue. This can also be done by blending plant tissue with other materials. On the other hand, decellularized plant tissue typically lacks mammalian biochemical cues, so it needs to be coated with compensatory functional proteins. C2C12 growth was not shown to change significantly between the bare scaffold and the same scaffold with a coating of collagen or gelatin proteins, however seeding efficiency (rate at which cells attach to the scaffold) improved. An advantage of decellularized plant tissue is the natural topography afforded by the leaf vasculature. This helps replicate the natural physiological state of the myoblasts which promotes cell alignment. The other ways of doing this are usually quite a bit more expensive including 3d printing, soft lithography and photolithography. Vascularization can also help overcome the 100–200 nm diffusion limit of culture medium into cells that usually produce necrotic centres in muscle conglomerates. Another way to do this is by having a porous scaffold which supports angiogenesis (the development of new blood vessels). While this has been shown to work for Apple Hypanthium, not all plants are nearly as porous. The alternative to plant cellulose is bacterial cellulose which is typically more pure than plant cellulose as it is free from contaminants such as lignin and hemicellulose. Bacterial cellulose has more hydrogen bonding between its polymer strands and so it has greater crystallinity. It also has smaller microfibrils that allow it to retain more moisture and have smaller pores. The substance can be produced using waste carbohydrates (which may allow it to be produced less expensively) and it adds juiciness and chewiness to emulsified meat (which would mean that even if it can't be taken out of the final product, it will contribute to the texture profile).[172][173]
Chitin
Chitin is nature's second most abundant polymer. It is found in the exoskeletons of crustaceans and fungi. As cellular agriculture is attempting to end reliance on animals, chitin derived from fungi is of greater interest. It has mostly been studied by Pelling Group. Chitosan is derived from chitin in a process known as alkaline deacetylation (substituting out certain amino acid groups). The degree of this process determines the physical and chemical properties of the chitosan. Chitosan has antibacterial properties; in particular, it has bactericidal effects on planktonic bacteria and biofilms and a bacteria static effects on gram negative bacteria such as E. coli. This is important as it neutralizes potentially harmful compounds without using antibiotics, which many consumers avoid. Chitosan's resemblance to glycosaminoglycans and internal interactions between glycoproteins and proteoglycans make it highly biocompatible. It can easily blend with other polymers in order to select for more bioactive factors. One potential disadvantage of chitosan is that it degrades in the presence of lysozymes (naturally occurring enzymes). But, this can be resisted using deacetylation. This is not entirely negative, as the byproducts produced through degradation have anti-inflammatory and anti-bacterial properties. It is important to match the level that cells rely on the matrix for structure with degradation.[172]
Collagen
Collagen is a family of proteins that makes up the primary structure of human connective tissue. It is typically derived from bovine, porcine and murine sources. Cellular agriculture overcomes this dependency through the use of transgenic organisms that are capable of producing the amino acid repeats that make up the collagen. Collagen naturally exists as collagen type I. It has been produced as porous hydrogels, composites and substrates with topographical cues and biochemical properties. Synthetic kinds of collagen have been produced through recombinant protein production—collagen type II and III, tropoelastin and fibronectin. One challenge with these proteins is that they can not be modified post translation. However, an alternative fibrillar protein has been isolated in microbes that lack collagen's biochemical cues, but has its kind of gene customizability. One focus of recombinant collagen production is yield optimization—how it can be produced most effectively. Plants, in particular, tobacco look like the best option, however, bacteria and yeast are also viable alternatives.[172]
Textured soy protein is a soy flour product often used in plant-based meat that supports the growth of bovine cells. Its spongy texture enables efficient cell seeding and its porosity encourages oxygen transfer. Additionally, it degrades during cell differentiation into compounds that are beneficial to certain cells.[174]
Mycellium
Mycelium are the roots of mushrooms. Altast Foods Co. is using solid state fermentation to grow mushroom tissue on mycelium scaffolds. They harvest this tissue and use it to create bacon analogs.[175]
Nanomaterials
Nanomaterials exhibit unique properties at the nanoscale. London-based Biomimetic Solutions is leveraging nanomaterials in order to create scaffolds.[174]
Cass Materials in Perth, Australia is using a dietary fibre called Nata de Coco (derived from coconuts) to create nanocellulose sponges for their BNC scaffold. Nata de Coco is biocompatible, has high porosity, facilitates cell adhesion and is biodegradable.[176]
Spinning
Immersion Jet Spinning is a method of creating scaffolds by spinning polymers into fibres, It was developed by the Parker Group at Harvard. Their platform uses centrifugal force to extrude a polymer solution through an opening in a rotating reservoir. During extrusion, the solution forms a jet that elongates and aligns as it crosses the air gap. The jet is directed into a vortex-controlled precipitation bath that chemically cross links or precipitates polymer nanofibers. Adjusting air gap, rotation and the solution changes the diameter of the resulting fibres. This method can spin scaffolds out of PPTA, nylon, DNA and nanofiber sheets. A nanofibrous scaffold made from alginate and gelatin was able to support the growth of C2C12 cells. Rabbit and bovine aortic smooth muscle myoblasts were able to adhere to the gelatin fibres. They formed aggregates on shorter fibres, and aligned tissue on the longer ones.[177]
Matrix Meats is using electrospinning—a process that uses electric force to turn charged polymers into fibres for scaffolds. Their scaffolds allowed meat marbling, are compatible with multiple cell lines, and are scalable.[178]
Additive manufacturing
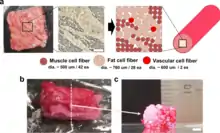
Another proposed way of structuring muscle tissue is additive manufacturing. Such a technique was perfected for industrial applications in manufacturing objects made out of plastic, nylon, metal, glass and other synthetic materials. The most common variation of the process involves incrementally depositing a filament in layers onto a bed until the object is completed. This method will most likely lend itself best to the application of cultured meat as opposed to other types such as binder jetting, material jetting or stereolithography that require a specific kind of resin or powder.
A filament of muscle cells can be printed into a structure meant to resemble a finished meat product which can then be further processed for cell maturation. This technique has been demonstrated in a collaboration between 3D bioprinting solutions and Aleph Farms that used additive manufacturing to structure turkey cells on the International Space Station.[180]
3D bioprinting has been used to produce steak-like cultured meat, composed of three types of bovine cell fibers and with a structure of assembled of cell fibers that is similar to original meat.[181][179]
Bioreactors
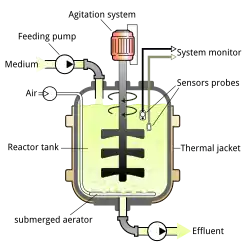
Scaffolds are placed inside bioreactors so that cell growth and specialization can occur. Bioreactors are large machines similar to brewery tanks which expose the cells to a large variety of environmental factors that are necessary to promote either proliferation or differentiation. The temperature of the bioreactor must replicate in vivo conditions. In the case of mammalian cells, this requires heating to 37 °C (99 °F). Alternatively, insect cells can be grown at room temperature. Most bioreactors are maintained at 5% carbon dioxide.[1][182]
Cells can either be cultivated in continuous or fed-batch systems. The former entails inoculating and harvesting cells in a constant process so that there are always cells in the bioreactor. Fed-batch systems mean inoculating the cells, culturing them and harvesting them in a single period.[1]
Stirred tank bioreactors are the most widely used configuration. An impeller increases the flow, thereby homogenizing the culture media and a diffuser facilitates the exchange of oxygen into the media. This system is generally used for suspended cultures but can be used for cells that require attachment to another surface if microcarriers are included. Fixed bed bioreactors are commonly used for adherent cultures. They feature strips of fibres that are packed together to form a bed to which cells can attach. Aerated culture media is circulated through the bed. In airlift bioreactors, the culture media is aerated into a gaseous form using air bubbles which are then scattered and dispersed amongst the cells. Perfusion bioreactors are common configurations for continuous cultivation. They continuously drain media saturated with lactic acid that is void of nutrients and fill it with replenished media.[183]
Fermentation
The elements outlined above apply to the cultivation of animal muscle tissue. However, cellular agriculture includes "acellular agriculture", which involves the production of animal products synthesized of non-living material. Such products include milk, honey, eggs, cheese, and gelatin which are made of various proteins rather than cells. In such cases, these proteins must be fermented much like in recombinant protein production, alcohol brewing and the generation of many plant-based products like tofu, tempeh and sauerkraut.[184]

Proteins are coded for by specific genes, the genes coding for the protein of interest are synthesized into a plasmid—a closed loop of double helical genetic information. This plasmid, called recombinant DNA, is then inserted into a bacterial specimen. For this to happen, the bacteria needs to be competent (i.e. able to accept foreign, extracellular DNA) and able to horizontally transfer genes (i.e. integrate the foreign genes into its own DNA). Horizontal gene transfer is significantly more challenging in eukaryotic organisms than prokaryotic organisms because the former have both a cell membrane and a nuclear membrane which the plasmid needs to penetrate whereas prokaryotic organisms only have a cell membrane. For this reason, prokaryotic bacteria are often favoured. In order to make such a bacteria temporarily competent, it can be exposed to a salt such as calcium chloride, which neutralizes the negative charges on the cell membrane's phosphate heads as well as the negative charges on the plasmid to prevent the two from repelling. The bacteria can incubate in warm water, opening large pores on the cell surface through which the plasmid can enter.[185]
Next, the bacteria is fermented in sugar, which encourages it to grow and duplicate. In the process it expresses its DNA as well as the transferred plasmid resulting in protein.
Finally, the solution is purified to separate out the residual protein. This can be done by introducing an antibody raised against the protein of interest that will kill bacteria cells that do not contain the protein. Through centrifugation, the solution can be spun around an axis with sufficient force to separate solids from liquids. Alternatively it could be soaked in a buffered ionic solution that employs osmosis to leach the water from bacteria and kill them.[186]
Challenges
Growth factors
The culture media is an essential component of in vitro cultivation. It is responsible for providing the macromolecules, nutrients and growth factors necessary for cell proliferation. Sourcing growth factors is one of the most challenging tasks of cellular agriculture. Traditionally, it involves the use of fetal bovine serum (FBS) which is a blood product extracted from fetal cows. Besides the argument that its production is unethical, it is also vitiates the independence of the use of animals. It is also the most costly constituent of cultured meat, priced at around $1000 per litre. Furthermore, chemical composition varies greatly depending on the animal, so it cannot be uniformly quantified chemically.[187] FBS is employed because it conveniently mimics the process of muscle development in vivo. The growth factors needed for tissue development are predominantly provided through an animal's bloodstream, and no other known fluid can single-handedly deliver all these components.[1]
The current alternative is to generate each growth factor individually using recombinant protein production. In this process, the genes coding for the specific factor are integrated into bacteria which are then fermented. However, due to the added complexity of this process, it is particularly expensive.[1] Future Fields, a Canadian company focused on overcoming the economic and environmental costs of traditional growth media, is developing serum-free growth factors from fruit flies. [188]
The ideal medium would be chemically quantifiable and accessible to ensure simplicity in production, cheap and not dependent on animals.[42] It will most likely be derived from plants and while this may reduce the possibility of transmitting infectious agents, it may induce allergic reactions in some consumers.[189] Such culture sera may also require modifications specific to the cell line to which it is applied. Companies currently invested in developing effective plant based culture includes Multus Media and Biftek.[190][191]
The Good Food Institute (GFI) put out a report in 2019 in support of the concept that cell-based meat could be produced at the same cost as ground beef and in 2021 they commissioned a report from CE Delft on the Techno-Economic Analysis of cultivated meat.[192]
Another approach is to subject the cell lines to a magnetic field, which stimulates the release of molecules that have regenerative, metabolic, anti-inflammatory and immunity-boosting properties, eliminating the need for serum.[193]
Surface area
A common challenge to bioreactors and scaffolds is developing system configurations that enable all cells to gain exposure to culture media while simultaneously optimizing spatial requirements. In the cell proliferation phase, prior to the introduction of the scaffold, many cell types need to be attached to a surface to support growth. As such, cells must be grown in confluent monolayers only one cell thick which necessitates a lot of surface area. This poses practical challenges on large scales. As such, systems may incorporate microcarriers—small spherical beads of glass or other compatible material that are suspended in the culture medium. Cells adhere to these microcarriers as they would to the sides of the bioreactor, which increases the amount of surface area.[194]
In the cell differentiation phase, the cells may be seeded to a scaffold and so do not require the use of microcarriers. However, in these instances, the density of the cells on the scaffold means that not all cells have an interface with culture media, leading to cell death and necrotic centers within the meat. When muscle is cultivated in vivo, this issue is circumvented as the extracellular matrix delivers nutrients into the muscle through blood vessels. As such, many emerging scaffolds aim to replicate such networks.[194]
Similarly, scaffolds must simulate many of the other characteristics of the extracellular matrix, most notably porosity, crystallinity, degradation, biocompatibility and functionality. Few materials that emulate all these characteristics have been identified, leading to the possibility of blending different materials with complementary properties.[172]
Research support
Cellular agriculture research does not have a significant basis of academic interest or funding streams.[19] Consequently, the majority of research has been undertaken and funded by independent institutions. However, this is incrementally changing as not for profits drive support and interest. Notably, New Harvest has a fellowship program to support graduate students and groups at various academic institutions.[195] However, a growing number of governments are funding research in cellular agriculture. In August 2020, the Grant Management Services of the European Commission awarded a €2.5 million grant to ORF Genetics.[196] In August 2020, the Japanese Ministry of Economy, Trade and Industry granted Integriculture $2.2 million through their New Energy and Industrial Technology Development Organization.[197] The European Union’s Horizon 2020 R&D funding framework awarded a €2.7 million grant to a consortium led by BioTech Foods.[198] In 2021, the Spanish government granted €3.7 million for Biotech Foods to investigate the potential health benefits of cellular agriculture.[199] The National Science Foundation awarded a $3.55 million grant to a team of researchers at UC Davis for open-access cultured meat research.[200] Non profits also drive support and interest in the field. Notably, New Harvest has a fellowship program to support the research of specific graduate students and groups at various academic institutions and the Good Food Institute funds open-access research through its Research Grant Program.
Consumer acceptance
Consumer acceptance of the product is critical.[201] A study looking at acceptance of cultured meat in China, India, and the US "found high levels of acceptance of clean meat in the three most populous countries worldwide."[202]
Several potential factors of consumer acceptance of cultured meat have been identified. Healthiness, safety, nutritional characteristics, sustainability, taste, and lower price, are all contributing factors.[203] One study found that the use of highly technical language to explain cultured meat led to significantly more negative public attitude towards the concept.[204] Transparently communicating the science is important, but oversharing the wrong aspects of the product could draw unfavourable attention to safety concerns.[205] Thus one of the challenges in how cultivated meat is marketed is striking the balance between transparency of the science behind it, but communicating it in a way that it does not evoke resistance.[206] One study suggested that describing cultured meat in a way that emphasizes the final product rather than the production method was an effective way to improve acceptance.[207] The role of nomenclature is also crucial. Although the 'lab-grown meat' portrayal of cultivated meat is favoured by media, it has been opposed by industry leaders as it seeds an innately unnatural image of cultivated meat in consumer's perceptions.[208]
The use of standardized descriptions would improve future research about consumer acceptance of cultured meat. Current studies have often reported drastically different rates of acceptance, despite similar survey populations.[209] Lou Cooperhouse, CEO of BlueNalu, shared on the Red to Green Podcast that "cell-based" and "cell-cultured" were suitable terms to differentiate it from conventional meat whilst being clear about the process by which it was made.[210] There also exists a challenge in how to use these descriptions in labelling. For example, in the United States there is no overarching federal legislation that regulates how cultured meat should be labeled for the consumer. While traditional meat producers are attempting to prevent cultured meat companies from using the term "meat," cultured meat producers argue that the word is necessary for consumer acceptance.[211]
Global market acceptance has not been assessed. Studies are attempting to determine the current levels of consumer acceptance and identify methods to improve this value. Clear answers are not available, although one recent study reported that consumers were willing to pay a premium for cultured meat.[212][213][204][214][203][207][215]
Low percentages of older adult populations have been reported to show acceptance for cultured meat. Green eating behavior, educational status, and food business, were cited as most important factors for this population.[214]
There is also a lack of studies relating the methods of producing cultured meat with its taste for the consuming public.
Regulations
Singapore became the first country in the world to approve cultured meat for sale in 2020. The Singapore Food Agency has published guidance on its requirements for the safety assessment of novel foods, including specific requirements on the information to be submitted for approval of cultivated meat products.[216]
Regulatory matters must also be sorted out. Prior to being available for sale, the European Union, Australia, New Zealand, the UK and Canada require approved novel food applications. Additionally, the European Union requires that cultured animal products and production must prove safety, by an approved company application, as of 1 January 2018.[217]
Within the United States, the FDA (Food and Drug Administration) and the USDA (United States Department of Agriculture) have agreed to jointly regulate cultured meat. Under the agreement, the FDA oversees cell collection, cell banks, and cell growth and differentiation, while the USDA oversees the production and labeling of human food products derived from the cells.[218]
Differences from conventional meat
Health
Large-scale production of cultured meat may or may not require artificial growth hormones to be added to the culture for meat production.[219][220]
As cultured meat is grown in a sterile environment, there is no need for antibiotics.[221] Today, the widespread use of antibiotics in conventional agriculture is the main driver of antibiotic resistance in humans.[222] According to the World Health Organization, antimicrobial resistance represents "an increasingly serious threat to global public health that requires action across all government sectors and society"[223] – predicting up to 10 million deaths annually by 2050.[224] Cultured meat could provide an effective solution to help mitigate this major risk to human health.
Researchers have suggested that omega-3 fatty acids could be added to cultured meat as a health bonus.[48] In a similar way, the omega-3 fatty acid content of conventional meat can be increased by altering what the animals are fed.[225] Research is currently underway in Spain to develop cultivated meat with healthier fats, which could reduce cholesterol and the risk of colon cancer typically associated with red meat consumption.[226] An issue of Time magazine suggested that the cell-cultured process may also decrease exposure of the meat to bacteria and disease.[49]
Due to the strictly controlled and predictable environment, cultured meat production has been compared to vertical farming. Some of its proponents have predicted that it will have similar benefits in terms of reducing exposure to dangerous chemicals like pesticides and fungicides, severe injuries, and wildlife.[227]
There is also a lack of research on the comparison on the health effects of production cultured meat with the industrial meat or the biologic organic meat ways of production.
Artificiality
Although cultured meat consists of animal muscle cells, fat and support cells, as well as blood vessels,[228] that are the same as in traditional meat, some consumers may find the high-tech production process unacceptable. Cultured meat has been described as fake or "Frankenmeat".[229] On the other hand, cultured meat can be produced without the artificial hormones, antibiotics, steroids, medicine, and GMOs commonly used in factory farmed meat and seafood, though not used on organic biologic production.
If a cultured meat product is different in appearance, taste, smell, texture, or other factors, it may not be commercially competitive with conventionally produced meat. The lack of bone and cardiovascular system is a disadvantage for dishes where these parts make appreciable culinary contributions. However, the lack of bones and/or blood may make many traditional meat preparations, such as buffalo wings, more palatable to some people. Furthermore, blood and bones could potentially be cultured in the future.[230][231][232]
Environment
Animal production for food is a major cause of air/water pollution and carbon emissions.[233] Significant questions have been raised about whether the traditional industry can meet the rapidly increasing demands for meat.[234] Cultured meat may provide an environmentally conscious alternative to traditional meat production.[235]
The environmental impacts of cultured meat are expected to be significantly lower than from animal husbandry.[236] For every hectare that is used for vertical farming and/or cultured meat manufacturing, anywhere between 10 and 20 hectares of land may be returned to its natural state.[237] Vertical farms (in addition to cultured meat facilities) could exploit methane digesters to generate a portion of its electrical needs. Methane digesters could be built on site to transform the organic waste generated at the facility into biogas which is generally composed of 65% methane. This biogas could be burned to generate electricity for the greenhouse or a series of bioreactors.[238]
One study reported that cultured meat was "potentially ... much more efficient and environmentally-friendly". It generated only 4% of greenhouse gas emissions, reduced the energy needs of meat production by up to 45%, and required only 2% of the land that the global meat/livestock industry does.[239][240] In Tuomisto's life cycle analysis claimed that producing 1000 kg of meat conventionally requires "26–33 GJ energy, 367–521 m3 water, 190–230 m2 land, and emits 1900–2240 kg CO2-eq GHG emissions". On the other hand, producing the same quantity of meat in vitro has "7–45% lower energy use... 78–96% lower GHG emissions, 99% lower land use, and 82–96% lower water use".[241]
The latest study by independent research firm CE Delft shows that—compared with conventional beef—cultured meat may cause up to 92% less greenhouse gas emissions if renewable energy is used in the production process, 93% less pollution, up to 95% less land use and 78% less water.[242]
Skeptic Margaret Mellon of the Union of Concerned Scientists speculates that the energy and fossil fuel requirements of large-scale cultured meat production may be more environmentally destructive than producing food off the land.[46] However, S.L. Davis speculated that both vertical farming in urban areas and the activity of cultured meat facilities may cause relatively little harm to the wildlife that live around the facilities.[243] Dickson Despommier speculated that natural resources may be spared from depletion due to vertical farming and cultured meat.[244] One study reported that conventional farming kills ten wild animals per hectare each year.[243]
Role of genetic modification
Techniques of genetic engineering, such as insertion, deletion, silencing, activation, or mutation of a gene, are not required to produce cultured meat. Cultured meat production allows the biological processes that normally occur within an animal to occur without the animal. Since cultured meat is grown in a controlled, artificial environment, some have commented that cultured meat more closely resembles hydroponic vegetables, rather than genetically modified vegetables.[245]
More research is underway on cultured meat, and although cultured meat does not require genetic engineering, researchers may employ such techniques to improve quality and sustainability. Fortifying cultured meat with nutrients such as beneficial fatty acids is one improvement that can be facilitated through genetic modification. The same improvement can be made without genetic modification, by manipulating the conditions of the culture medium.[246] Genetic modification may be able to enhance muscle cell proliferation. The introduction of myogenic regulatory factors, growth factors, or other gene products into muscle cells may increase production over that of conventional meat.[246]
To avoid the use of any animal products, the use of photosynthetic algae and cyanobacteria has been proposed to produce the main ingredients for the culture media, as opposed to fetal bovine or horse serum.[247] Some researchers propose that the ability of algae and cyanobacteria to produce ingredients for culture media can be improved with certain technologies, most likely not excluding genetic engineering.[248]
Ethical
Australian bioethicist Julian Savulescu said, "Artificial meat stops cruelty to animals, is better for the environment, could be safer and more efficient, and even healthier. We have a moral obligation to support this kind of research. It gets the ethical two thumbs up."[249] Animal welfare groups are generally in favor of cultured meat, because the culture process does not include a nervous system and therefore does not involve pain or infringement of rights.[46][250][251] Reactions of vegetarians to cultured meat vary.[252] Some feel the cultured meat presented to the public in August 2013 was not vegetarian because fetal bovine serum was used in the growth medium.[253] However, since then, cultured meat has been grown with a medium that does not involve bovine serum.[254] Philosopher Carlo Alvaro argues that the question of the morality of eating in vitro meat has been discussed only in terms of convenience. Alvaro proposes a virtue-oriented approach, suggesting that the determination to produce cultured meat stems from unvirtuous motives, i.e., "lack of temperance and misunderstanding of the role of food in human flourishing."[255]
Some have proposed independent inquiries into the standards, laws, and regulations for cultured meat.[256]
Just as with many other foods, cultured meat needs technically sophisticated production methods that may be difficult for some communities, meaning they would lack self-sufficiency and be dependent on global food corporations.[257] However, some projects are focusing on making cell ag accessible to all. The open source cellular agriculture initiative Shojinmeat Project has for instance a bottom-up approach, teaching members of the project to cultivate DIY cultured meat at home.[258]
Establishing a similar parallel with cultured meat, some environmental activists claim that adopting a vegetarian diet may be a way of focusing on personal actions and righteous gestures rather than systemic change. Environmentalist Dave Riley states that "being meatless and guiltless seems seductively simple while environmental destruction rages around us", and notes that Mollison "insists that vegetarianism drives animals from the edible landscape so that their contribution to the food chain is lost".[259]
Religious considerations
Jewish rabbinical authorities disagree whether cultured meat is kosher, meaning acceptable under Jewish law and practice. One factor is the nature of the animal from which the cells are sourced, whether it is a kosher or non-kosher species and whether, if the cells were taken from a dead animal, slaughter in accordance with religious practice had taken place prior to the extraction of cells. Most authorities agree that if the original cells were taken from a religiously slaughtered animal then the meat cultured from it will be kosher.[260] Depending on the nature of the cells, it may be determined to be kosher even when taken from a live animal, and some have argued that it would be kosher even if coming from non-kosher animals such as pigs.[22]
Islamic dietary practices must also be considered.[261] The Islamic Institute of Orange County, California, said, "There does not appear to be any objection to eating this type of cultured meat."[262] In addition, Abdul Qahir Qamar of the International Islamic Fiqh Academy said that cultured meat "will not be considered meat from live animals, but will be cultured meat." As long as the cells are not from pigs, dogs, and other haram animals, the meat would be considered vegetative and "similar to yogurt and fermented pickles."[262]
Hinduism typically excludes the consumption of beef, such as steak and burgers. Chandra Kaushik, president of the Hindu Mahasabha, said about cultured beef that he would "not accept it being traded in a marketplace in any form or being used for a commercial purpose."[262]
Catholicism, which excludes eating meat in certain days along the year (Lent, Holy Week), has not pronounced on whether cultivated meat is banned (as it happens with meat) or not (as with any other food as vegetables or fish).
Economic
At the moment, cultured meat is significantly more costly than conventional meat. However, in a March 2015 interview, Post said that the marginal cost of his team's original €250,000 burger was now €8.00. He estimated that technological advancements would allow the product to be cost-competitive to traditionally sourced beef in approximately ten years.[263] In 2018, Memphis Meats reduced the cost of production to $1,700 per pound.[153] In 2019, Eat Just said it cost about US$50 to produce one chicken nugget.[264] The company’s cultured chicken nuggets, now available at Singapore restaurant 1880, cost around US$17.[265] A study by independent research firm CE Delft found that production costs for cultivated meat could fall to $5.66/kg by 2030.[266]
Farmers
A scientific paper published in Front. Sustain. Food Syst. addresses the social and economic opportunities and challenges of cultured and plant-based meat for rural producers. According to this research, cellular agriculture offers "opportunities such as growing crops as ingredients for feedstock for cultured meat; raising animals for genetic material for cultured meat; producing cultured meat in bioreactors at the farm level; transitioning into new sectors; new market opportunities for blended and hybrid animal- and alt-meat products; and new value around regenerative or high-animal welfare farming." However, some challenges are also identified, with possible "loss of livelihood or income for ranchers and livestock producers and for farmers growing crops for animal feed; barriers to transitioning into emerging alt-meat sectors; and the possibility of exclusion from those sectors." Some farmers already see the potential of cellular agriculture. For instance, Illtud Dunsford comes from a long line of farmers in Wales and established his cultured meat company Cellular Agriculture Ltd in 2016.[267]
Continuing development
Education
In 2015, Maastricht University hosted the first International Conference on Cultured Meat.[268] New Harvest[269]—a 501(c)(3) research institute—as well as The Good Food Institute[270] host annual conferences to convene industry leaders, scientists, investors, and potential collaborators. The two organizations also fund public research and produce educational content. Organizations such as the Cellular Agriculture Society, Cellular Agriculture Canada, Cellular Agriculture France, Cellular Agriculture Australia and Cellular agriculture New Zealand were founded to advocate for cultured meat in their respective countries. Publications such as Cell Agri and the Protein Report have also emerged in order to provide updates concerning the technology and business within the field.
Research
Research continues on many fronts, including entomoculture, interactome maps of cardiac tissue,[271] substrate design,[271] scaffold design,[271] nutritional profile,[271] reaction kinetics, transport phenomena, mass transfer limitations and metabolic stoichiometric requirements,[271] and bioprinting process.[271]
Accelerators and incubators
Multiple venture capital firms and accelerator/incubator programs focus on assisting cultured technology startups, or plant-based protein companies in general. The Big Idea Ventures (BIV) Venture Capital firm launched their New Protein Fund to invest in emerging cell and plant-based food companies in New York and Singapore. They invested in MeliBio, Actual Veggies, Biftek.co, Orbillion Bio, Yoconut, Evo, WildFor and Novel Farms.[272] Indie Bio is a biology oriented accelerator program that has invested in Memphis Meats, Geltor, New Age Meats and Finless Foods.[273]
In popular culture
Cultured meat has often featured in science fiction. The earliest mention may be in Two Planets (1897) by Kurd Lasswitz, where "synthetic meat" is one of the varieties of synthetic food introduced on Earth by Martians. Other notable books mentioning artificial meat include Ashes, Ashes (1943) by René Barjavel; The Space Merchants (1952) by Frederik Pohl and C.M. Kornbluth; The Restaurant at the End of the Universe (1980) by Douglas Adams; Le Transperceneige (Snowpiercer) (1982) by Jacques Lob and Jean-Marc Rochette; Neuromancer (1984) by William Gibson; Oryx and Crake (2003) by Margaret Atwood; Deadstock (2007) by Jeffrey Thomas; Accelerando (2005) by Charles Stross; Ware Tetralogy by Rudy Rucker; Divergent (2011) by Veronica Roth; and the Vorkosigan Saga (1986–2018) by Lois McMaster Bujold.
In film, artificial meat has featured prominently in Giulio Questi's 1968 drama La morte ha fatto l'uovo (Death Laid an Egg) and Claude Zidi's 1976 comedy L'aile ou la cuisse (The Wing or the Thigh). "Man-made" chickens also appear in David Lynch's 1977 surrealist horror, Eraserhead. Most recently, it was also featured prominently as the central theme of the movie Antiviral (2012).
The Starship Enterprise from the TV and movie franchise Star Trek apparently provides a synthetic meat,[274] although crews from The Next Generation and later use replicators.
In the ABC sitcom Better Off Ted (2009–2010), the episode "Heroes" features Phil (Jonathan Slavin) and Lem (Malcolm Barrett) trying to grow cowless beef.[275]
In the videogame Project Eden, the player characters investigate a cultured meat company called Real Meat.
In the movie Galaxy Quest during the dinner scene, Tim Allen's character refers to his steak tasting like "real Iowa beef".
In The Expanse "vat-grown" meat is produced to feed the people who live on spaceships/space stations away from Earth, due to the exorbitant cost of importing real meat.
Cultured meat was a subject on an episode of the Colbert Report on 17 March 2009.[276]
In February 2014, a biotech startup called BiteLabs ran a campaign to generate popular support for artisanal salami made with meat cultured from celebrity tissue samples.[277] The campaign became popular on Twitter, where users tweeted at celebrities asking them to donate muscle cells to the project.[278] Media reactions to BiteLabs variously identified the startup as a satire on startup culture,[279] celebrity culture,[280] or as a discussion prompt on bioethical concerns.[281] While BiteLabs claimed to be inspired by the success of Sergey Brin's burger, the company is seen as an example of critical design rather than an actual business venture.
In late 2016, cultured meat was involved in a case in the episode "How The Sausage Is Made" of CBS show Elementary.[282]
Cultured meat was profiled in the 2020 Canadian documentary film Meat the Future.[283]
In the 2020 video game Cyberpunk 2077, multiple cultured meat products are for sale, due to the high cost of natural meat. This includes "EEZYBEEF", made from in vitro cultured muscle cells taken from cattle, and the flatworm culture based "Orgiatic" which comes in several flavors.
See also
- BioArt
- BioTech Foods
- Cellular agriculture society
- Factory farming divestment
- Food vs. feed
- Cultured leather
- List of meat substitutes
- Meat analogue
- Quorn (food product)
- Resource decoupling
- Shark fin soup substitute
- Timeline of cellular agriculture
- Tissue culture
- Veganism
References
- Datar, I (January 2010). "Possibilities for an in vitro meat production system". Innovative Food Science & Emerging Technologies. 11 (1): 13–22. doi:10.1016/j.ifset.2009.10.007.
- De Lorenzo, Daniela (17 March 2022). "Dutch Parliament Approves Cultured Meat Tasting In The Netherlands". Forbes.com. Retrieved 8 April 2022.
- Post, Mark (4 December 2013). "Medical technology to Produce Food". Journal of the Science of Food and Agriculture. 94 (6): 1039–1041. doi:10.1002/jsfa.6474. PMID 24214798.
- Edelman, PD (3 May 2005). "Commentary: In Vitro-Cultured Meat Productionsystem". Tissue Engineering. 11 (5–6): 659–662. CiteSeerX 10.1.1.179.588. doi:10.1089/ten.2005.11.659. PMID 15998207. Retrieved 8 April 2018.
- Schonwald, Josh (May 2009). "Future Fillet". The University of Chicago Magazine.
- Bryant, Christopher J (3 August 2020). "Culture, meat, and cultured meat". Journal of Animal Science. 98 (8): skaa172. doi:10.1093/jas/skaa172. ISSN 0021-8812. PMC 7398566. PMID 32745186.
- Hong, Tae Kyung; Shin, Dong-Min; Choi, Joonhyuk; Do, Jeong Tae; Han, Sung Gu (May 2021). "Current Issues and Technical Advances in Cultured Meat Production: AReview". Food Science of Animal Resources. 41 (3): 355–372. doi:10.5851/kosfa.2021.e14. ISSN 2636-0772. PMC 8112310. PMID 34017947.
- Treich, Nicolas (1 May 2021). "Cultured Meat: Promises and Challenges". Environmental and Resource Economics. 79 (1): 33–61. doi:10.1007/s10640-021-00551-3. ISSN 1573-1502. PMC 7977488. PMID 33758465.
- Bryant, Christopher J (1 August 2020). "Culture, meat, and cultured meat". Journal of Animal Science. 98 (8): skaa172. doi:10.1093/jas/skaa172. PMC 7398566. PMID 32745186.
- Treich, Nicolas (May 2021). "Cultured Meat: Promises and Challenges". Environmental and Resource Economics. 79 (1): 33–61. doi:10.1007/s10640-021-00551-3. PMC 7977488. PMID 33758465.
- Kolyohin, Nick (2 July 2021). "Feature: Israeli cultured meat company aims to redefine industry". Xinhua News Agency. Retrieved 2 July 2021.
- Peters, Adele (5 November 2020). "At the first lab-grown meat restaurant, you can eat a 'cultured chicken' sandwich". Fast Company. Retrieved 18 January 2021.
- Scully, Matthew (17 January 2021). "Hello Cultured Meat, Goodbye to the Cruelty of Industrial Animal Farming". National Review. Retrieved 18 January 2021.
- "What is the most consumed meat in the world?". Retrieved 14 October 2021.
- "Investors eat up Orbillion Bio's plans for lab-grown Wagyu beef, elk and bison". 26 April 2021. Retrieved 14 October 2021.
- "Lab-grown fish makes a debut in Hong Kong". 29 January 2021. Retrieved 14 October 2021.
- "Seafood Without The Sea: Will Lab-Grown Fish Hook Consumers?". 5 May 2019. Retrieved 14 October 2021.
- "Future Food - In Vitro Meat". futurefood.org. November 2018. Retrieved 26 November 2018.
- Rohrheim, A (June 2016). "Cultured Meat". Sentience Politics. Archived from the original on 1 December 2018. Retrieved 26 November 2018.
- Zaraska, Marta (19 August 2013). "Is Lab-Grown Meat Good for Us?". The Atlantic.
- Anthis, Jacy Reese (19 October 2018). "Slaughter-Free Meat Is An Answer To Our Cruel And Broken Food System". The Huffington Post. Retrieved 10 April 2019.
- JTA. "Rabbi: Lab-grown pork could be kosher for Jews to eat – with milk". Times Of Israel. Retrieved 22 March 2018.
- Fountain, Henry (6 August 2013). "A Lab-Grown Burger Gets a Taste Test". The New York Times. Retrieved 2 February 2016.
- "USDA and FDA to Host Joint Meeting On Cell-Based Meat Regulation". VegNews.com. Retrieved 26 November 2018.
- Banis, Davide (14 December 2018). "7 Predictions On The Future Of Clean Meat in 2019". Forbes. Retrieved 10 April 2019.
- Watson, Elaine (12 September 2019). "'Cultivated' meat could be the most-consumer-friendly term for cell-cultured meat, suggests Mattson/GFI research". FoodNavigator-USA.
- Jha, Alok (5 August 2013). "Synthetic meat: how the world's costliest burger made it on to the plate". The Guardian. Retrieved 2 February 2016.
- "Bill Gates wants you to eat artificial meat".
- ""Clean Meat": The "Clean Energy" of Food". 6 September 2016.
- ""Clean Meat," "Cell-Based Meat," "Slaughter-Free Meat": How We Talk About Meat Grown without Animals". The Good Food Institute. 27 September 2018. Retrieved 14 October 2019.
- "Lab-made meat rebranded 'clean meat' to address 'yuck' factor". GlobalMeatNews.
- ""Clean meat" is catching on: a reflection on nomenclature". The Good Food Institute. 24 May 2018. Archived from the original on 16 September 2018. Retrieved 5 June 2018.
- "Cultured meat cos agree to replace term 'clean meat' with 'cell-based meat' and form trade association". foodnavigator-usa.com. Retrieved 14 October 2019.
- "'Cell-based meat' not the most consumer-friendly term, reveals GFI consumer research". foodnavigator-usa.com. Retrieved 14 October 2019.
- Friedrich, Bruce (13 September 2019). "Cultivated Meat: Why GFI Is Embracing New Language". The Good Food Institute. Retrieved 14 October 2019.
- Friedrich, Bruce (29 September 2021). "Cultivated meat: A growing nomenclature consensus". The Good Food Institute. Archived from the original on 1 October 2021. Retrieved 9 October 2021.
- "No Bull". - International Churchill Society
- Purdy, Chase (24 September 2017). "The idea for lab-grown meat was born in a prisoner-of-war camp". Quartz. Archived from the original on 24 September 2017. Retrieved 9 February 2021.
- Specter, Michael (16 May 2011). "Test-Tube Burgers". The New Yorker. Retrieved 9 February 2021.
{{cite magazine}}: CS1 maint: url-status (link) - Frey, Thomas (30 May 2019). "The Future of the Cultured Meats Industry in 2040". Futurist Speaker. Retrieved 20 November 2019.
- WO application 9931222, van Eelen, Willem Frederik; van Kooten, Willem Jan & Westerhof, Wiete, "Industrial scale production of meat from in vitro cell cultures", published 1999-06-24
- Kadim, Isam T; Mahgoub, Osman; Baqir, Senan; Faye, Bernard; Purchas, Roger (February 2015). "Cultured meat from muscle stem cells: A review of challenges and prospects". Journal of Integrative Agriculture. 14 (2): 222–233. doi:10.1016/S2095-3119(14)60881-9.
- Shapiro, Paul (19 December 2017). "Lab-Grown Meat Is on the Way". Scientific American: Observations. Retrieved 20 November 2019.
- Catts, Oron; Zurr, Ionat (Winter 2004–2005). "Ingestion / Disembodied Cuisine". Cabinet Magazine.
- "Paper Says Edible Meat Can be Grown in a Lab on Industrial Scale" (Press release). University of Maryland. 6 July 2005. Archived from the original on 25 July 2005. Retrieved 12 October 2008.
- Levine, Ketzel (20 May 2008), "Lab-Grown Meat a Reality, But Who Will Eat It?", NPR.org, National Public Radio, retrieved 10 January 2010
- "PETA's 'In Vitro' Chicken Contest". PETA. 6 October 2008. Retrieved 5 December 2019.
- Macintyre, Ben (20 January 2007). "Test-tube meat science's next leap". The Australian. Archived from the original on 2 November 2011. Retrieved 26 November 2011.
- Siegelbaum, D.J. (23 April 2008). "In Search of a Test-Tube Hamburger". Time. Archived from the original on 22 January 2010. Retrieved 30 April 2009.
- "The 50 Best Inventions of 2009". Time. 12 November 2009. Archived from the original on 15 November 2009.
- Rogers, Lois (29 November 2009). "Scientists grow pork meat in a laboratory". The Sunday Times. London. Archived from the original on 6 January 2010. Retrieved 8 December 2009.
- "World's first lab-grown burger is eaten in London". BBC News. 5 August 2013. Retrieved 2 February 2016.
- Fountain, Henry. "Engineering the $325,000 In Vitro Burger". Retrieved 12 June 2018.
- Hogenboom, Melissa (5 August 2013). "What does a stem cell burger taste like?". BBC News. Retrieved 2 February 2016.
- "Kweekvlees en vleesvervangers - Rondetafelgesprek 26-9-2018". Arnews (in Dutch). Dutch House of Representatives. 26 September 2018. Retrieved 23 October 2018.
- "Memphis Meats rebrands as UPSIDE Foods, gears up to launch cell-cultured chicken by year-end: 'This is a big historic step for the entire industry'". foodnavigator-usa.com. Retrieved 5 January 2022.
- Bunge, Jacob (1 February 2016). "Sizzling Steaks May Soon Be Lab-Grown". The Wall Street Journal. Retrieved 4 February 2016.
- "'World's first' lab-grown meatball revealed". Fox News. Retrieved 4 February 2016.
- Addady, Michal (2 February 2016). "You Could Be Eating Lab-Grown Meat in Just Five Years". Fortune. Retrieved 4 February 2016.
- Bunge, Jacob (15 March 2017). "Startup Serves Up Chicken Produced From Cells in Lab". The Wall Street Journal. Retrieved 17 March 2017.
- Farber, Madeline (15 March 2017). "A San Francisco Startup Is Serving Chicken That Was Made in a Lab". Fortune. Retrieved 17 March 2017.
- Kooser, Amanda. "This lab-grown chicken and duck meat looks surprisingly delicious March 15, 2017". CNET. Retrieved 17 March 2017.
- Chang, Lulu (11 July 2016). "SuperMeat wants you to try its lab-grown chicken breast". Digital Trends.
- "Lab-Grown Chicken Could Soon Be On Your Plate". Sky News. 12 July 2016. Retrieved 5 August 2016.
- Chang, Lulu (11 July 2016). "Would you eat lab grown chicken? SuperMeat sure hopes so". Yahoo News. Retrieved 5 August 2016.
- Tobin, Andrew (13 July 2016). "The Israeli Startup That Lets You Eat Meat - Without Eating the Animal". Haaretz. Retrieved 5 August 2016.
- Tobin, Andrew (13 July 2016). "No harm, no fowl: Startup to grow chickenless chicken". The Times of Israel. Retrieved 5 August 2016.
- Card, Jon (24 July 2017). "Lab-grown food: 'the goal is to remove the animal from meat production'". The Guardian. Retrieved 13 January 2018.
- Mac van Dinther (31 March 2018). "Een écht stukje vlees, zonder dat daar dode dieren aan te pas komen: het komt eraan". de Volkskrant (in Dutch). Retrieved 20 May 2018.
- Gijs Vroom (4 March 2020). "Krijn de Nood (Meatable): 'Wij pionieren een nieuwe manier van vlees maken'". Emerce. Retrieved 26 May 2020.
- Brodwin, Erin (28 September 2018). "A new lab-grown meat startup may have overcome a key barrier to making meat without slaughter". Business Insider. Retrieved 29 September 2018.
- Shieber, Jonathan. "Future Fields is tackling cultured meat's biggest problem". TechCrunch. Retrieved 12 February 2021.
- Evich, Helena Bottemiller (29 August 2019). "Cell-based meat companies join forces". Politico. Retrieved 14 October 2019.
- Purdy, Chase (29 August 2019). "Cell-cultured meat companies just created a brand-new lobbying group". Quartz. Retrieved 14 October 2019.
- "Meatable becomes a founding member of newly created association for cellular agriculture". Meatable.com. Meatable. 3 December 2021. Retrieved 10 December 2021.
- "Cellular Agriculture Europe Launches to Become the "Voice of the Cultivated Industry"". Vegconomist. 7 December 2021. Retrieved 10 December 2021.
- Amy Buxton (8 December 2021). "Cellular Agriculture Europe Offers a Powerful Voice to the Cultivated Meat, Seafood, and Ingredients Industry". Green Queen. Retrieved 10 December 2021.
- Dieter De Cleene (17 December 2019). "Vlaanderen investeert in kweekvlees". Eos Wetenschap (magazine) (in Dutch). Retrieved 26 May 2020.
- Yves Degroote (8 May 2020). "Belgisch bedrijf bouwt 2 labo's voor kweekvlees". VTM (in Dutch). Retrieved 27 May 2020.
- Bert Broens, Jens Cardinaels (14 May 2021). "Plannen voor Belgische proeffabriek voor kweekvet". De Tijd (in Dutch). Retrieved 7 December 2021.
- Smithers, Rebecca (7 October 2019). "First meat grown in space lab 248 miles from Earth". Guardian News & Media Limited. The Guardian. Retrieved 12 July 2020.
- Purdy, Chase (22 January 2020). "A startup says it's building a US pilot plant for cell-based meat". Quartz. Retrieved 27 May 2020.
- Purdy, Chase (13 May 2020). "As the US meat supply chain fumbles, cultured meat startups consider a better system". Quartz. Retrieved 27 May 2020.
- "Accelerating the cultured meat revolution". New Scientist. 20 May 2020. Retrieved 27 May 2020.
- "Chinese Official Calls for National Strategy to Allow China to Keep up With Other Countries Making Progress in Cultured Meat". Vegconomist. 25 June 2020. Retrieved 12 August 2020.
- "Indian Cell-Based Meat Company Claims it Has Achieved Price Parity". Vegconomist. 30 November 2020. Retrieved 8 December 2021.
- Oliver Morrison. "'Everybody accepts the cultured meat trend is happening': Biotech Foods". Food Navigator. Retrieved 24 July 2020.
- Oliver Holmes (4 December 2021). "I tried the world's first no-kill, lab-grown chicken burger". The Guardian. Retrieved 6 December 2021.
- Jonah Mandel (23 June 2021). "Lab-grown chicken 'food revolution' gathers pace at Ness Ziona eatery". Times of Israel. Retrieved 6 December 2021.
- Damian Carrington (2 December 2020). "No-kill, lab-grown meat to go on sale for first time". The Guardian. Retrieved 2 December 2020.
- Jade Scipioni (18 December 2020). "This restaurant will be the first ever to serve lab-grown chicken (for $23)". CNBC. Retrieved 6 December 2021.
- Andrew Noyes (21 December 2020). "Eat Just Makes History (Again) with Restaurant Debut of Cultured Meat". Business Wire. Retrieved 6 December 2021.
- "UE approva raccolta firme per togliere i sussidi agli allevamenti e incentivare la carne vegetale e coltivata". Fanpage.it (in Italian).
- "As Meat Shortages Spread Globally, These 6 Startups Offer Alternative Cuts". Calcalistech. 10 May 2020. Retrieved 22 August 2020.
- Dieter De Cleene (12 November 2019). "Wanneer ligt kweekvlees op ons bord?". Eos Wetenschap (magazine) (in Dutch). Retrieved 26 May 2020.
- "Aleph Farms' Space Program Has Lift Off as the Cultivated Steak Leader Reveals New HQ". Vegconomist. 17 February 2022. Retrieved 12 May 2022.
- "'At first it seemed like science fiction…' Cell-cultured meat startup Artemys Foods emerges from stealth mode". foodnavigator-usa.com. Retrieved 22 October 2020.
- Sito, Peggy (17 August 2020). "Hong Kong protein alternatives start-up eyes juicy cut of sector forecast to reach US$630 billion by 2040". South China Morning Post. Retrieved 11 October 2020.
- "Avant Meats Has First Public Taste Test of Cultured Fish Maw in Hong Kong". The Spoon. 25 November 2019. Retrieved 22 October 2020.
- "Lab Grown and Cultured Meat - Balletic Foods". balleticfoods.com.
- "Pets may soon be fed laboratory-grown meat". The Economist. 28 January 2021. ISSN 0013-0613. Retrieved 19 May 2021.
- "Read about our cultured meat cat treat". Because Animals. Retrieved 19 May 2021.
- Peters, Adele (16 August 2021). "There are now lab-grown mouse-meat cookies for cats". Fast Company. Retrieved 27 October 2021.
- "Biftek.co". biftek.co.
- "Turkey's first lab-grown meat on its way". TRT World. 28 November 2019.
- "In Texas, BioBQ is Betting on Brisket as the Next Big Thing for Cell-Based Meat". The Spoon. 12 October 2020. Retrieved 22 October 2020.
- "BlueNalu just months from first batch of yellowtail, mahi mahi grown from cells". Undercurrent News. Retrieved 22 October 2020.
- "JBS takes over Bio Tech Foods". AgrarZeitung. 7 December 2021. Retrieved 8 December 2021.
- Vincent West (10 July 2019). "The €250,000 lab-grown burger could be a more palatable €9 in two years". Irish Independent. Reuters. Retrieved 7 December 2020.
- Agnieszka de Sousa (10 December 2020). "Lab-Grown Meat Is Getting Closer to Supermarket Shelves". Bloomberg. Retrieved 8 December 2021.
[Aleph Farms Ltd.] is also working on a pilot plant. Companies such as BioTech Foods, SuperMeat and Eat Just have already started testing sites.
- "3D-Printed Meat Startup CellX Raises $4.3 Million in Funding". 3Dnatives. 13 September 2021. Retrieved 4 October 2021.
- Chow, Emily; Patton, Dominique (4 September 2021). "Chinese firm serves up lab-grown pork in world's top meat market". Reuters. Retrieved 4 October 2021.
- Chow, Emily; Patton, Dominique. "Chinese firm serves up lab-grown pork". STLtoday.com. Reuters. Retrieved 4 October 2021.
- "A Chinese Biotech Startup Could Best Conventional Meat Prices by 2025". interestingengineering.com. 1 October 2021. Retrieved 4 October 2021.
- Deepsekhar Choudhury (25 January 2020). "Clear Meat seeks to solve the problem of animal slaughter through its cell-based meat culture". Financial Express. Retrieved 8 December 2021.
- "ClearMeat, India: "Our Target is to Accelerate Clean Meat Adoption in India"". Vegconomist. 6 May 2019. Retrieved 8 December 2021.
- "Smart Fat: Novel technology to transform liquid oils into solid fats could boost juiciness in plant-based meats, reduce sat fat, claims Cubiq Foods". foodnavigator-usa.com. Retrieved 22 October 2020.
- Joint ventrue of Migros, Givaudan and Bühler. "Migros, Givaudan und Bühler gründen ein Labor für kultiviertes Fleisch". Luzerner Zeitung (in German). 15 September 2021. Retrieved 13 December 2021.
- Corbyn, Zoë (19 January 2020). "Out of the lab and into your frying pan: the advance of cultured meat". The Guardian. Retrieved 26 May 2020.
- "Kweekvlees is er, maar het eten mag nog niet". Nieuwsuur (in Dutch). NOS. 22 May 2018. Retrieved 26 May 2020.
- Kowitt, Beth (19 December 2017). "Silicon Valley and the Search for Meatless Meat". Fortune. Retrieved 26 May 2020.
- "Inside the Quest to Make Lab Grown Meat". Wired. 16 February 2018. Retrieved 22 August 2020.
- Alex Barreira (14 October 2021). "The cell-cultivated meat revolution is starting, and these Bay Area startups are ready". San Francisco Business Times. Retrieved 6 December 2021.
- Kateman, Brian (17 February 2020). "Will Cultured Meat Soon Be A Common Sight In Supermarkets Across The Globe?". Forbes. Retrieved 26 May 2020.
- Ally Dunne (23 June 2021). "Future Meat Technologies Launches World's First Industrial Cultured Meat Production Facility". PR Newswire. Retrieved 6 December 2021.
- Shieber, Jonathan (10 October 2019). "Lab-grown meat could be on store shelves by 2022, thanks to Future Meat Technologies". TechCrunch. Retrieved 26 May 2020.
- Edwards, Charlotte (27 July 2020). "Higher Steaks Makes World's First Cell-Based Pork Belly". Green Queen. Retrieved 21 August 2020.
- Petzinger, Jill (21 July 2020). "UK startup Higher Steaks creates world's first lab-grown bacon prototype". Yahoo Finance UK. Retrieved 21 August 2020.
- Nakamura, Keita (15 July 2019). "Start-up dreams of feeding world with cheap lab-cultured meat". Kyodo News. Kyodo News. Retrieved 14 July 2020.
- Marvell, Helen (5 June 2018). "Japanese Government Part of $2.7 Million Investment in Slaughter-Free Foie Gras". LIVEKINDLY. Retrieved 22 October 2020.
- "Cell-cultured meat gamechanger? Matrix Meats to showcase nanofiber scaffolding in 'solid meat product' this year". foodnavigator-usa.com. Retrieved 22 October 2020.
- Till Behne (24 April 2021). "Delftse slimmeriken Daan en Krijn komen in 2025 met kweekvlees: 'We willen voorop lopen'". Algemeen Dagblad (in Dutch). Retrieved 9 December 2021.
- Pepijn de Lange (13 September 2021). "DSM stapt in kweekvlees met start-up Meatable: groen, diervriendelijk en (hopelijk) rendabel". de Volkskrant (in Dutch). Retrieved 9 December 2021.
- Watson, Elaine (11 February 2020). "Cell-based meat in focus: In conversation with Meatable, Finless Foods, New Age Meats". Food Navigator. Retrieved 21 August 2020.
- "Peace of Meat: "We gaan 100.000 ton kweekvet per jaar produceren"". Kweekvlees.be (in Dutch). GAIA. 3 June 2020. Retrieved 7 December 2021.
- Schuller, Jil (8 June 2020). "Swiss start-up: laboratory meat from Zurich". Bauernzeitung (in German). Retrieved 22 August 2020.
- Leonie Hosselet (6 February 2017). "Van het lab naar een bord is een lange weg voor kweekvlees". Trouw (in Dutch). Retrieved 26 May 2020.
- "Mosa Meats Announces it Has Reduced Production Costs by 88 Times". Vegconomist. 23 July 2020. Retrieved 20 August 2020.
- Martine Kamsma (7 February 2020). "De race om kweekvlees". NRC Handelsblad (in Dutch). Retrieved 25 May 2020.
- Splitter, Jenny (15 August 2019). "This New Animal-Free Ingredient Company Just Raised Another $27.5 Million In Funding". Forbes. Retrieved 11 October 2020.
- "Biotech: The future of food". Business Daily. 27 August 2020. Retrieved 11 October 2020.
- Shieber, Jonathan (1 October 2020). "Motif FoodWorks preps commercial production for its first ingredient". TechCrunch. Retrieved 11 October 2020.
- "Student team tackling unsustainability of meat industry win Imperial competition | Imperial News | Imperial College London". Imperial News. Retrieved 22 October 2020.
- "New Age Meats Raises $2M Seed Extension to Continue Developing Cultivated Pork". PR Newswire. 30 July 2020. Retrieved 22 August 2020.
- Brodwin, Erin; Canales, Katie (22 September 2018). "We tried the first lab-grown sausage made without killing animals. It was smoky, savory, and tasted like breakfast". Business Insider. Retrieved 22 August 2020.
- Sally Ho (4 October 2021). "New Age Meats To Launch Cell-Based Sausages By 2022 After $25M Series A". Green Queen. Retrieved 7 December 2021.
- Yu, Doris (24 June 2020). "Singapore's Shiok Meats bags $3m in bridge funding ahead of series A". Tech in Asia. Retrieved 21 August 2020.
- David Pierson (8 October 2020). "The first lab-grown meat for sale could come from this Singapore startup that's re-creating shrimp". Los Angeles Times. Retrieved 10 October 2020.
Shiok’s proto-shrimp costs $5,000 a kilogram, which is about $2,268 a pound, mostly due to the price of the nutrient fluids needed to feed the cells. Access to more affordable nutrients has reduced the cost of Shiok’s meat to $3,500 a kilogram, or about $1,588 a pound. (...) Shiok’s shumai, for example, cost $300 apiece. (...) The goal is to make Shiok’s shrimp 100 times cheaper by the first half of next year.
- Silverberg, David (24 March 2020). "Could synthetic fish be a better catch of the day?". BBC News. Retrieved 21 August 2020.
- Aravindan, Aradhana; Teo, Travis (28 January 2020). "Singapore's Shiok Meats hopes to hook diners with lab-grown shrimp". Reuters. Retrieved 21 August 2020.
- "Default Parallels Plesk Panel Page". shojinmeat.com.
- "SuperMeat founder: 'The first company that gets to market with cultured meat that is cost effective is going to change the world'". foodnavigator-usa.com. Retrieved 22 October 2020.
- Chris Dart (4 May 2020). "Documentary 'Meat the Future' shows us the possible future of meat". Canadian Broadcasting Corporation. Retrieved 26 May 2020.
- Clifford, Catherine (24 August 2017). "Why Richard Branson, Bill Gates and Jack Welch all invested in this start-up that grows meat in a lab". CNBC. Retrieved 26 May 2020.
- Katie Spalding (8 November 2021). "World's Most Advanced Lab-Grown Meat Facility Opens In California". IFLScience. LabX Media Group. Retrieved 5 December 2021.
- Brian Kateman (30 November 2021). "11 Plant-Based And Alternative Protein Trends To Watch For In 2022". Forbes. Retrieved 5 December 2021.
- Cherney, Mike (8 August 2019). "Lab-grown kangaroo meat: it's what's for dinner?". Wall Street Journal. Retrieved 10 October 2020.
- Klar, Michal (20 November 2019). "Cell-based meat startups in Asia-Pacific show prototypes and raise funding". Get Revue. Retrieved 10 October 2020.
- Sier, Jessica (2 October 2022). "The new factory making meat that's illegal to eat". Australian Financial Review. Retrieved 6 October 2022.
- Bronner, Stephen J. (24 October 2019). "Lab-grown meat also creates an unexpected benefit: ethical zebra burgers". Inverse. Retrieved 10 October 2020.
- David Yaffe-Bellany (10 July 2019). "The Fish Is Boneless. (Fishless, Too.)". The New York Times. Retrieved 6 December 2021.
- Alex Barreira (25 June 2021). "Sushi grown in a lab? A S.F. startup just unveiled the world's first cell-cultured seafood production facility". San Francisco Business Times. Retrieved 6 December 2021.
- Gülsöken, Deniz. "Slaughter-Free Food Is The New And The Now Way Of Feeding The World". Forbes. Retrieved 7 January 2022.
- Catherine Lamb (20 June 2018). "CAS Wants You (and Everyone Else) to Know About Cellular Agriculture". The Spoon. Retrieved 24 October 2020.
- "Impossible Foods: Meat made from plants". impossiblefoods.com.
- Ricky Ben-David (25 November 2021). "Lab to table: Israeli tech kitchens cook up future of animal-free food". The Times of Israel. Retrieved 5 December 2021.
- Sally Ho (20 September 2021). "MeaTech Is Now Churning Out 100% Pure Cultured Chicken Fat". Green Queen. Retrieved 7 December 2021.
- Louis Gore Langton (19 October 2020). "Food for thought: cultured meat maker brings in $55m in funding". DutchNews.nl. Retrieved 6 December 2021.
- Flora Southey (25 September 2020). "How will Mosa Meat spend its latest $55m injection?". Food Navigator. Retrieved 6 December 2021.
- "Frequently asked questions about stem cell research". Mayo Clinic. Retrieved 17 October 2020.
- "Induced Pluripotent Stem Cells (iPS) | UCLA Broad Stem Cell Center". stemcell.ucla.edu. Retrieved 17 October 2020.
- Campuzano, Santiago; Pelling, Andrew E. (2019). "Scaffolds for 3D Cell Culture and Cellular Agriculture Applications Derived From Non-animal Sources". Frontiers in Sustainable Food Systems. 3. doi:10.3389/fsufs.2019.00038. ISSN 2571-581X. S2CID 157058210.
- Adamski, Michal; Fontana, Gianluca; Gershlak, Joshua R.; Gaudette, Glenn R.; Le, Hau D.; Murphy, William L. (31 May 2018). "Two Methods for Decellularization of Plant Tissues for Tissue Engineering Applications". Journal of Visualized Experiments (135). doi:10.3791/57586. ISSN 1940-087X. PMC 6101437. PMID 29912197.
- Ben-Arye, Tom; Shandalov, Yulia; Ben-Shaul, Shahar; Landau, Shira; Zagury, Yedidya; Ianovici, Iris; Lavon, Neta; Levenberg, Shulamit (April 2020). "Textured soy protein scaffolds enable the generation of three-dimensional bovine skeletal muscle tissue for cell-based meat". Nature Food. 1 (4): 210–220. doi:10.1038/s43016-020-0046-5. ISSN 2662-1355. S2CID 216199677.
- "Atlast Food Co". Atlast Food Co. Retrieved 18 October 2020.
- "Cass Materials". Retrieved 18 October 2020.
- Gonzalez, Grant M.; MacQueen, Luke A.; Lind, Johan U.; Fitzgibbons, Stacey A.; Chantre, Christophe O.; Huggler, Isabelle; Golecki, Holly M.; Goss, Josue A.; Parker, Kevin Kit (2017). "Production of Synthetic, Para-Aramid and Biopolymer Nanofibers by Immersion Rotary Jet-Spinning". Macromolecular Materials and Engineering. 302 (1): 1600365. doi:10.1002/mame.201600365. ISSN 1439-2054.
- "Matrix Meats". Matrix Meats. Retrieved 18 October 2020.
- Kang, Dong-Hee; Louis, Fiona; Liu, Hao; Shimoda, Hiroshi; Nishiyama, Yasutaka; Nozawa, Hajime; Kakitani, Makoto; Takagi, Daisuke; Kasa, Daijiro; Nagamori, Eiji; Irie, Shinji; Kitano, Shiro; Matsusaki, Michiya (24 August 2021). "Engineered whole cut meat-like tissue by the assembly of cell fibers using tendon-gel integrated bioprinting". Nature Communications. 12 (1): 5059. Bibcode:2021NatCo..12.5059K. doi:10.1038/s41467-021-25236-9. ISSN 2041-1723. PMC 8385070. PMID 34429413.
- "Home". MeaTech.
- "Japanese scientists produce first 3D-bioprinted, marbled Wagyu beef". New Atlas. 25 August 2021. Retrieved 21 September 2021.
- Rubio, Natalie R.; Fish, Kyle D.; Trimmer, Barry A.; Kaplan, David L. (2019). "Possibilities for Engineered Insect Tissue as a Food Source". Frontiers in Sustainable Food Systems. 3. doi:10.3389/fsufs.2019.00024. ISSN 2571-581X. S2CID 116877741.
- "What Are the Different Types of Bioreactors? - Biotech Blog". www.pall.com. Retrieved 19 October 2020.
- "What is Cellular Agriculture?". New Harvest. 16 August 2016. Retrieved 28 October 2020.
- "CaCl2 Transformation Technique". MyBioSource Learning Center. Retrieved 28 October 2020.
- Wingfield, Paul T. (1 April 2015). "Overview of the Purification of Recombinant Proteins". Current Protocols in Protein Science. 80: 6.1.1–6.1.35. doi:10.1002/0471140864.ps0601s80. ISSN 1934-3655. PMC 4410719. PMID 25829302.
- Dessels, Carla; Potgieter, Marnie; Pepper, Michael S. (2016). "Making the Switch: Alternatives to Fetal Bovine Serum for Adipose-Derived Stromal Cell Expansion". Frontiers in Cell and Developmental Biology. 4: 115. doi:10.3389/fcell.2016.00115. ISSN 2296-634X. PMC 5065960. PMID 27800478.
- "Future Fields Cellular Agriculture & Biomanufacturing". www.futurefields.io. Retrieved 28 October 2020.
- I. Datar, M. Betti, Possibilities for an in vitro meat production system, Innovative Food Science and Emerging Technologies 11 (2010) at 17.
- "Multus | feeding food". multus.media. Retrieved 28 October 2020.
- "biftek.co". biftek.co. Retrieved 28 October 2020.
- "TEA of cultivated meat. Future projections for different scenarios". CE Delft - EN. Retrieved 7 January 2022.
- Andrei, Mihai (4 October 2022). "New technique from Singapore makes lab-grown meat cheaper, greener, and more ethical". ZME Science. Retrieved 12 October 2022.
- "How it's made: the science behind cultivated meat". A Bit of Science. Retrieved 19 October 2020.
- "New Harvest". New Harvest. Retrieved 19 October 2020.
- "Icelandic Biotech Firm Receives Large European Grant". Iceland Monitor. Retrieved 7 January 2022.
- "IntegriCulture Awarded $2.2 Million Grant to Build New Commercial Cell Ag Facility". The Spoon. 28 August 2020. Retrieved 7 January 2022.
- "EU's Horizon 2020 invests in cellular agriculture". Food Frontier. 14 October 2020. Retrieved 7 January 2022.
- "BioTech Food tests on synthetic meat for € 5.2 million - Spanish government allocates 3.7 million for the project". EFA News - European Food Agency. 22 January 2021. Retrieved 7 January 2022.
- "UC Davis receives $3.55m grant to investigate long-term viability of cell-cultured meat". foodnavigator-usa.com. Retrieved 7 January 2022.
- Sharma, Shruti; Thind, Sukhcharanjit Singh; Kaur, Amarjeet (December 2015). "In vitro meat production system: why and how?". Journal of Food Science and Technology. 52 (12): 7599–7607. doi:10.1007/s13197-015-1972-3. ISSN 0022-1155. PMC 4648904. PMID 26604337.
- Bryant, Christopher; Szejda, Keri; Parekh, Nishant; Desphande, Varun; Tse, Brian (27 February 2019). "A Survey of Consumer Perceptions of Plant-Based and Clean Meat in the USA, India, and China". Frontiers in Sustainable Food Systems. 3: 11. doi:10.3389/fsufs.2019.00011. ISSN 2571-581X.
- Gómez-Luciano, Cristino Alberto; de Aguiar, Luis Kluwe; Vriesekoop, Frank; Urbano, Beatriz (December 2019). "Consumers' willingness to purchase three alternatives to meat proteins in the United Kingdom, Spain, Brazil and the Dominican Republic" (PDF). Food Quality and Preference. 78: 103732. doi:10.1016/j.foodqual.2019.103732. S2CID 198753926.
- Bryant, Christopher; Dillard, Courtney (3 July 2019). "The Impact of Framing on Acceptance of Cultured Meat". Frontiers in Nutrition. 6: 103. doi:10.3389/fnut.2019.00103. ISSN 2296-861X. PMC 6616100. PMID 31334244.
- 3.4. Startup vs. Corporate: stop bashing animal agriculture & the importance of industry acceptance with Jack A Bobo, 5 May 2021, retrieved 4 August 2021
- "3.3. Chris Bryant - University of Bath". Red To Green Podcast. Retrieved 4 August 2021.
- Siegrist, Michael; Sütterlin, Bernadette; Hartmann, Christina (May 2018). "Perceived naturalness and evoked disgust influence acceptance of cultured meat". Meat Science. 139: 213–219. doi:10.1016/j.meatsci.2018.02.007. PMID 29459297.
- 3.6. Branding cultured products: the naturalness trap with Nicky Quinn, Global Marketing Director of Aleph Farms, 19 May 2021, retrieved 4 August 2021
- Bryant, Christopher; Barnett, Julie (September 2018). "Consumer acceptance of cultured meat: A systematic review". Meat Science. 143: 8–17. doi:10.1016/j.meatsci.2018.04.008. PMID 29684844. S2CID 19086898.
- 3.7. Dairy and fish vs. cultured meat: the difference in perception, production and promotion with Raffael Wolgensinger CEO of FORMO and Lou Cooperhouse CEO of BlueNalu, 26 May 2021, retrieved 4 August 2021
- Spring, Alexandra; Bence, Cydnee (18 October 2022). "WHAT'S THE BEEF? DEBATES OVER CELL-CULTURED MEAT" (PDF). Labels Unwrapped. Retrieved 25 October 2022.
- Bryant, Christopher J.; Anderson, Joanna E.; Asher, Kathryn E.; Green, Che; Gasteratos, Kristopher (August 2019). "Strategies for overcoming aversion to unnaturalness: The case of clean meat". Meat Science. 154: 37–45. doi:10.1016/j.meatsci.2019.04.004. PMID 30986669. S2CID 117717742.
- Bryant, Christopher J.; Barnett, Julie C. (June 2019). "What's in a name? Consumer perceptions of in vitro meat under different names". Appetite. 137: 104–113. doi:10.1016/j.appet.2019.02.021. PMID 30840874. S2CID 73479055.
- Grasso, Alessandra C.; Hung, Yung; Olthof, Margreet R.; Verbeke, Wim; Brouwer, Ingeborg A. (15 August 2019). "Older Consumers' Readiness to Accept Alternative, More Sustainable Protein Sources in the European Union". Nutrients. 11 (8): 1904. doi:10.3390/nu11081904. ISSN 2072-6643. PMC 6723411. PMID 31443177.
- Valente, Júlia de Paula Soares; Fiedler, Rodrigo Alonso; Sucha Heidemann, Marina; Molento, Carla Forte Maiolino (30 August 2019). Gao, Zhifeng (ed.). "First glimpse on attitudes of highly educated consumers towards cell-based meat and related issues in Brazil". PLOS ONE. 14 (8): e0221129. Bibcode:2019PLoSO..1421129V. doi:10.1371/journal.pone.0221129. ISSN 1932-6203. PMC 6716657. PMID 31469862.
- "SFA" (PDF). Archived (PDF) from the original on 4 December 2020.
- Stephens, N (21 July 2018). "Bringing cultured meat to market: Technical, socio-political, and regulatory challenges in cellular agriculture". Trends in Food Science & Technology. 78: 155–166. doi:10.1016/j.tifs.2018.04.010. PMC 6078906. PMID 30100674.
- "USDA/FDA Launches Joint Webinar on Roles and Responsibilities for Cultured Animal Cell Human and Animal Food Products". United States Food and Drug Administration. FDA Center for Food Safety and Applied Nutrition. 9 September 2020.
- Edelman, P. D; McFarland, D. C.; Mironov, V. A.; Matheny, J. G. (2005). "In vitro-cultured meat production". Tissue Engineering. 11 (5–6): 659–662. doi:10.1089/ten.2005.11.659. PMID 15998207.
- Marta Zaraska (19 August 2013). "Is Lab-Grown Meat Good for Us?". The Atlantic. Retrieved 2 February 2016.
- "Could lab-grown meat prevent the next pandemic?". ZME Science. 14 January 2021. Retrieved 12 January 2022.
- Martin, Michael J.; Thottathil, Sapna E; Newman, Thomas B. (December 2015). "Antibiotics Overuse in Animal Agriculture: A Call to Action for Health Care Providers". American Journal of Public Health. 105 (12): 2409–2410. doi:10.2105/AJPH.2015.302870. ISSN 0090-0036. PMC 4638249. PMID 26469675.
- "Antibiotic resistance". www.who.int. Retrieved 12 January 2022.
- "UN, global health agencies sound alarm on drug-resistant infections; new recommendations to reduce 'staggering number' of future deaths". UN News. 29 April 2019. Retrieved 12 January 2022.
- Azcona, J.O.; Schang, M.J.; Garcia, P.T.; Gallinger, C.; Ayerza, R. (2008). "Omega-3 enriched broiler meat: The influence of dietary alpha-linolenic omega-3 fatty acid sources on growth, performance and meat fatty acid composition". Canadian Journal of Animal Science, Ottawa, Ontario, Canada. 88 (2): 257–269. doi:10.4141/cjas07081.
- "Spanish government invests €5.2 million in cultured meat project". foodnavigator.com. Retrieved 12 January 2022.
- Despommier, D. (2008). "Vertical Farm Essay I". Vertical Farm. Archived from the original on 1 July 2009. Retrieved 26 June 2009.
- "World's first lab-grown steak is made from beef but slaughter-free". 18 December 2018.
- Kerr, Dara (19 February 2016). "Lab-grown food: It's what's for dinner!". CNET. Retrieved 8 July 2017.
- "Lab-Grown Blood To Be Trialled in the U.K." IFLScience.
- Bradley, Sian (12 September 2017). "How do you grow bone in a lab? Good vibrations". Wired UK.
- Pigott, George M.; Tucker, Barbee W. (1990). Seafood. CRC Press. p. 236. ISBN 978-0-8247-7922-1.
- "How Eating Less Meat Could Help Protect the Planet From Climate Change". Time. Retrieved 5 December 2019.
- Morris, Regan; Cook, James (15 October 2018). "Would you eat slaughter-free meat?". Retrieved 5 December 2019.
- Rodríguez Fernández, Clara (18 December 2018). "You Will Be Eating Lab-Grown Meat Soon: Here's What You Need to Know". Labiotech.eu. Retrieved 5 December 2019.
- Tuomisto, Hannah (17 June 2011), "Environmental Impacts of Cultured Meat Production", Environmental Science & Technology, 45 (14): 6117–6123, Bibcode:2011EnST...45.6117T, doi:10.1021/es200130u, PMID 21682287
- A Farm on Every Floor, The New York Times, 23 August 2009
- Case Study – Landfill Power Generation Archived 3 December 2008 at the Wayback Machine, H. Scott Matthews, Green Design Initiative, Carnegie Mellon University. Retrieved 07.02.09
- Specter, Michael (23 May 2011), "Annals of Science, Test-Tube Burgers", The New Yorker, retrieved 28 June 2010
- Lab-grown meat would 'cut emissions and save energy', 21 June 2011
- Tuomisto, Hanna (17 June 2011). "Environmental Impacts of Cultured Meat Production". Environmental Science & Technology. 45 (14): 6117–6123. Bibcode:2011EnST...45.6117T. doi:10.1021/es200130u. PMID 21682287. Retrieved 12 November 2020.
- "CE_Delft_LCA_of_cultivated_meat.pdf". Google Docs. Retrieved 12 January 2022.
- S.L. Davis (2001). "The least harm principle suggests that humans should eat beef, lamb, dairy, not a vegan diet". Proceedings of the Third Congress of the European Society for Agricultural and Food Ethics. pp. 449–450.
- Despommier, Dickson (November 2009). "The Rise of Vertical Farms". Scientific American. 301 (5): 60–67. Bibcode:2009SciAm.301e..80D. doi:10.1038/scientificamerican1109-80. ISSN 0036-8733. PMID 19873908.
- Sandhana, Lakshmi. "Test Tube Meat Nears Dinner Table". Archived from the original on 19 August 2013. Retrieved 27 January 2014.
- Vein, John. "Patent US6835390". Retrieved 27 January 2014.
- Haagsman, H.P.; K.J. HelIingwerf; B.A.J. Roelen (October 2009). "Production of Animal Proteins by Cell Systems" (PDF). Universiteit Utrecht: Faculty of Veterinary Medicine: 13–14. Archived from the original (PDF) on 12 November 2013. Retrieved 27 January 2014.
- Tuomisto, Hanna L.; Teixeira de Mattos, M. J. (22–24 September 2010). "Life cycle assessment of cultured meat production" (PDF): 5. Archived from the original on 3 February 2014. Retrieved 27 January 2014.
{{cite journal}}: Cite journal requires|journal=(help) - Alok Jha (5 August 2013). "Synthetic meat: how the world's costliest burger made it on to the plate". The Guardian. Retrieved 2 February 2016.
- Raizel, Robin (11 December 2005). "In Vitro Meat". The New York Times. Retrieved 7 August 2009.
- Kruglinski, Susan; Wright, Karen (22 September 2008). "I'll Have My Burger Petri-Dish Bred, With Extra Omega-3". Discover.
- Izundu, Chi Chi (23 February 2012). "Could vegetarians eat a 'test tube' burger?". BBC News. Retrieved 2 February 2016.
- Hines, Nico (7 August 2013). "Can Vegetarians Eat In-Vitro Meat? The Debate Rages". The Daily Beast. Retrieved 2 February 2016.
- "A new lab-grown meat startup may have overcome a key barrier to making meat without slaughter". UK Business Insider. 28 September 2018. Retrieved 28 September 2018.
- Alvaro, C. (2019) Lab‐Grown Meat and Veganism: A Virtue‐Oriented Perspective. J Agric Environ Ethics. https://doi.org/10.1007/s10806-019-09759-2, p. 17.
- In vitro meat Archived 21 November 2011 at the Wayback Machine at Food Ethics Council
- "In Vitro Meat: Power, Authenticity and Vegetarianism". Archived from the original on 5 August 2013. Retrieved 5 August 2013.
- "Shojinmeat is Growing a DIY Clean Meat Community". The Spoon. 16 April 2018. Retrieved 12 January 2022.
- "Does meat make the meal?". 5 September 2016.
- Kenigsberg, Joel; Zivotofsky, Ari (22 January 2020). "A Jewish Religious Perspective on Cellular Agriculture". Frontiers in Sustainable Food Systems. 3. doi:10.3389/fsufs.2019.00128.
- Hamdan, Mohammad Naqib; Post, Mark J.; Ramli, Mohd Anuar; Mustafa, Amin Rukaini (1 December 2018). "Cultured Meat in Islamic Perspective". Journal of Religion and Health. 57 (6): 2193–2206. doi:10.1007/s10943-017-0403-3. ISSN 1573-6571. PMID 28456853. S2CID 9217711.
- "But Is It Kosher?". HuffPost. 9 August 2013. Retrieved 5 December 2019.
- Post, Mark (26 March 2015). "Mark Post of Maastricht University in the Netherlands has developed synthetic beef patties". Australian Broadcasting Corporation. Retrieved 14 May 2015.
- Morrison, Oliver (4 December 2020). "'A major milestone for lab-grown meat': Could Eat Just's approval in Asia hurry the market in Europe?". foodnavigator.com. William Reed Business Media. Retrieved 10 April 2021.
- Gilchrist, Karen (1 March 2021). "This multibillion-dollar company is selling lab-grown chicken in a world-first". CNBC. Retrieved 12 January 2022.
- "LCA of cultivated meat. Future projections for different scenarios". CE Delft - EN. Retrieved 12 January 2022.
- "Artificial meat: UK scientists growing 'bacon' in labs". BBC News. 19 March 2019. Retrieved 12 January 2022.
- "International Conference on Cultured Meat 2015". Cultured Beef. Retrieved 10 April 2019.
- Albrecht, Chris (20 July 2018). "Catch Video from the New Harvest Cultured Meat Conference". The Spoon. Retrieved 10 April 2019.
- "The Good Food Conference". The Good Food Conference. Retrieved 10 April 2019.
- "Current Research Projects". New Harvest. Retrieved 21 October 2020.
- Marston, Jennifer (13 August 2020). "Meet the 13 Companies Chosen for Cohort II of Big Idea Ventures' Alt-Protein Accelerator". The Spoon. Retrieved 28 October 2020.
- "Companies". IndieBio. Retrieved 28 October 2020.
- "Star Trek 'Charlie X'".
- Fresco, Michael (25 March 2009), Heroes, Better Off Ted, retrieved 25 October 2022
- "The Colbert Report: World of Nahlej – Shmeat". Comedy Central. 17 March 2009. Retrieved 1 December 2016.
- "BiteLabs". bitelabs.org.
- Jauregui, Andres (3 March 2014). "Hunger Game? Startup Whets Public Appetite For Salami Made From Celebrities". The Huffington Post.
- Merchant, Brian (26 February 2014). "The Guy Who Wants to Sell Lab-Grown Salami Made of Kanye West Is "100% Serious"". Motherboard. Vice.
- Knibbs, Kate (28 February 2014). "No, This Website Won't Actually Make Salami Out Of Famous People". Time.
- Harris, Jenn (5 March 2014). "Ellen DeGeneres salami? One company's quest to make meat from celebrity tissue samples". Los Angeles Times.
- "Elementary - How the Sausage is Made - Review: "Fake Meat, Real Murder"". www.spoilertv.com. Retrieved 25 October 2022.
- Dorothy Woodend, "Are We Ready to ‘Meat the Future’?". The Tyee, May 15, 2020.

