Guaifenesin
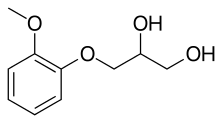
Guaifenesin', sold under the brand name Mucinex, among others,[3] is a medication used as an expectorant, intended to help cough out phlegm from the airways.[3] Chemically it is an ether of guaiacol and glycerine. It is unclear if it decreases coughing.[3] Use is not recommended in children less than six years old.[4] It is often used in combination with other medications.[3] It is taken by mouth.[3]
 | |
| Clinical data | |
|---|---|
| Pronunciation | /ɡwaɪˈfɛnɪsɪn/[1] |
| Trade names | Mucinex, others |
| Other names | Glyceryl guaiacolate |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682494 |
| License data |
|
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Metabolism | Kidney |
| Elimination half-life | 1–5 hours[2] |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.002.021 |
| Chemical and physical data | |
| Formula | C10H14O4 |
| Molar mass | 198.218 g·mol−1 |
| 3D model (JSmol) | |
| Chirality | Racemic mixture |
| |
| |
| (verify) | |
Side effects may include dizziness, sleepiness, skin rash, and nausea.[3] While it has not been properly studied in pregnancy, it appears to be safe.[5] It is believed to work by making airway secretions more liquid.[3]
Guaifenesin has been used medically since at least 1933.[6] It is available as a generic medication and over the counter.[3][5] In 2020, it was the 324th most commonly prescribed medication in the United States, with more than 800 thousand prescriptions.[7][8]
Medical use
Guaifenesin is used to try to help with coughing up thick mucus and is sometimes combined with dextromethorphan, an antitussive (cough suppressant), such as in Mucinex DM or Robitussin DM.[9] It is also combined with ephedrine to produce Primatene and Bronkaid tablets for symptomatic relief of asthma.
Side effects
Side-effects of guaifenesin include nausea, vomiting, formation of kidney stones,[10] diarrhea, and constipation.[11] Nausea and vomiting can be reduced by taking guaifenesin with meals.[3] The risk of forming kidney stones during prolonged use can be reduced by maintaining good hydration and increasing the pH of urine. Rarely, severe allergic reactions may occur, including a rash or swelling of the lips or gums, which may require urgent medical assistance. Mild dry mouth or chapped lips may also occur when taking this medication. Drinking a glass of water is recommended with each dose of guaifenesin.[12]
Pharmacology
Mechanism of action
Guaifenesin is thought to act as an expectorant by increasing the volume and reducing the viscosity of secretions in the trachea and bronchi. It may aid in the flow of respiratory tract secretions, allowing ciliary movement to carry the loosened secretions upward toward the pharynx.[13] Thus, it may increase the efficiency of the cough reflex and facilitate removal of the secretions.
Guaifenesin has muscle relaxant and anticonvulsant properties and may act as an NMDA receptor antagonist.[14]
History
Similar medicines derived from the guaiac tree were in use as a generic remedy by American indigenous peoples when explorers reached North America in the 16th century. The Spanish encountered guaiacum wood "when they conquered Santo Domingo; it was soon brought back to Europe, where it acquired an immense reputation in the sixteenth century as a cure for syphilis and certain other diseases..."[15]
The 1955 edition of the Textbook of Pharmacognosy states: "Guaiacum has a local stimulant action which is sometimes useful in sore throat. The resin is used in chronic gout and rheumatism, whilst the wood is an ingredient in the compound concentrated solution of sarsaparilla, which was formerly much used as an alternative in syphilis."[15]
In the US, guaifenesin was first approved by the Food and Drug Administration (FDA) in 1952. Although previously deemed "Generally Regarded as Safe" in its original approval, the drug received a New Drug Application for the extended-release version, which received approval on 12 July 2002.[16] Because of this, the FDA then issued letters to other manufacturers of timed-release guaifenesin to stop marketing their unapproved versions, leaving Adams Respiratory Therapeutics in control of the market. In 2007, Adams was acquired by Reckitt Benckiser.[17][18] The drug is sold over-the-counter by many companies, alone and in combination.[19]
Availability
Guaifenesin is taken by mouth.[3] It is available under many brand names, as either the lone active ingredient or as one part of a combination drug. Drugs combined with guaifenesin in over-the-counter preparations include the cough-suppressant dextromethorphan, analgesics such as paracetamol/acetaminophen, and decongestants such as ephedrine, pseudoephedrine, or phenylephrine.
Veterinary use
Guaifenesin's neurological properties first became known in the late 1940s. Guaifenesin is a centrally acting muscle relaxant used routinely in large-animal veterinary surgery. Guaifenesin is used in combination with, for example, ketamine, since guaifenesin does not provide analgesia nor does it produce unconsciousness.[20][21]
Research
The guaifenesin protocol was studied as a method to treat fibromyalgia; a one-year double-blind study found that the treatment performs no better than placebo.[22][23]
Guaifenesin was studied as a possible fertility medication, working by thinning and increasing the stretchability (spinnbarkeit) of the cervical mucus, during the few days before ovulation, thus facilitating sperm penetration.[24][25]
Results from a 2014 study by the Virginia Commonwealth University's Department of Pediatrics indicated that guaifenesin did not have significant impact on sputum production or clearance in upper respiratory infections.[26] This was consistent with a 2014 study involving 378 adult and adolescent participants, which indicated guaifenesin had no significant effect as either mucolytic or expectorant compared to placebo: "Although the upper respiratory quality of life improved over the course of the study in both the [guaifenesin] and placebo groups, this improvement was not accompanied by changes in sputum properties".[27]
References
- "Guaifenesin Definition & Meaning". Merriam-Webster.
- Aluri JB, Stavchansky S (1993). "Determination of guaifenesin in human plasma by liquid chromatography in the presence of pseudoephedrine". J Pharm Biomed Anal. 11 (9): 803–808. doi:10.1016/0731-7085(93)80072-9. PMID 8218524.
- "Guaifenesin Monograph for Professionals". Drugs.com. American Society of Health-System Pharmacists. Retrieved 25 March 2019.
- British national formulary : BNF 76 (76 ed.). Pharmaceutical Press. 2018. p. 295. ISBN 9780857113382.
- Weiner CP, Rope K (2013). The Complete Guide to Medications During Pregnancy and Breastfeeding: Everything You Need to Know to Make the Best Choices for You and Your Baby. St. Martin's Press. p. PT282. ISBN 9781250037206.
- Riviere JE, Papich MG (2013). Veterinary Pharmacology and Therapeutics. John Wiley & Sons. p. 287. ISBN 9781118685907.
- "The Top 300 of 2020". ClinCalc. Retrieved 31 October 2022.
- "Guaifenesin – Drug Usage Statistics". ClinCalc. Retrieved 31 October 2022.
- "Guaifenesin DM". WebMD.com.
- Bennett S, Hoffman N, Monga M (December 2004). "Ephedrine- and guaifenesin-induced nephrolithiasis". J Altern Complement Med. 10 (6): 967–9. doi:10.1089/acm.2004.10.967. PMID 15673990.
- Guaifenesin Side Effects https://www.drugs.com/sfx/guaifenesin-side-effects.html
- "Guaifenesin". MedlinePlut. United States Library of Medicine.
- Gutierrez, K. (2007). Pharmacotherapeutics: Clinical Reasoning in Primary Care. W.B. Saunders Co.
- Keshavarz M, Showraki A, Emamghoreishi M (2013). "Anticonvulsant Effect of Guaifenesin against Pentylenetetrazol-Induced Seizure in Mice". Iran J Med Sci. 38 (2): 116–21. PMC 3700057. PMID 23825891.
- Wallis TE (1955). Textbook of Pharmacognosy.
- "Drug Approval Package: Mucinex (Guaifenesin) NDA #21-282". accessdata.fda.gov. 25 November 2002. Retrieved 26 October 2022.
- "Announcements RB Press release - 10/12/2007". Archived from the original on 15 July 2011. Retrieved 16 November 2010.
- Goldstein J (25 May 2007). "FDA Bumps Phlegm-Fighters From Market". The Wall Street Journal. Retrieved 16 November 2010.
- "Guaifenesin (Oral Route) Description and Brand Names". Mayo Clinic. Retrieved 27 July 2021.
- Tranquilli WJ, Thurmon JC, Grimm KA, eds. (2007). "Centrally Acting Muscle Relaxants". Lumb and Jones' Veterinary Anesthesia and Analgesia (2nd ed.). Blackwell Publishing.
- Valverde A (April 2013). "Balanced anesthesia and constant-rate infusions in horses". Vet Clin North Am Equine Pract. 29 (1): 89–122. doi:10.1016/j.cveq.2012.11.004. PMID 23498047.
- "Consumer Alert — Guaifenesin for Fibromyalgia". Fmnetnews.com. Archived from the original on 23 October 2011. Retrieved 4 January 2012.
- Bennett RM, De Garmo P, Clark SR (1996). "A Randomized, Prospective, 12 Month Study To Compare The Efficacy Of Guaifenesin Versus Placebo In The Management Of Fibromyalgia" (reprint). Arthritis and Rheumatism. 39 (Supplement9): S212. doi:10.1002/art.1780391402.
- Weschler T (2002). Taking Charge of Your Fertility (Revised ed.). New York: HarperCollins. p. 52. ISBN 0-06-093764-5.
- Check JH, Adelson HG, Wu CH (May 1982). "Improvement of cervical factor with guaifenesin". Fertility and Sterility. 37 (5): 707–708. doi:10.1016/s0015-0282(16)46287-4. PMID 6896190.
- O'Connell, Oisin (May 2014). "Is Extended-Release Guaifenesin No Better Than a Placebo?". Respiratory Care. 59 (5): 788–789. doi:10.4187/respcare.03319. PMID 24789023. S2CID 31421167.
- Hoffer-Schaefer, Agathe (May 2014). "Guaifenesin Has No Effect on Sputum Volume or Sputum Properties in Adolescents and Adults With Acute Respiratory Tract Infections". Respiratory Care. 59 (5): 631–636. doi:10.4187/respcare.02640. PMID 24003241. S2CID 37309835. Retrieved 30 August 2021.
External links
- "Guaifenesin". Drug Information Portal. U.S. National Library of Medicine.
- "F.D.A. Study Worries Makers of Drugs". The New York Times. 20 October 1981.