Pyrazinamide
Pyrazinamide is a medication used to treat tuberculosis.[2] For active tuberculosis, it is often used with rifampicin, isoniazid, and either streptomycin or ethambutol.[3] It is not generally recommended for the treatment of latent tuberculosis.[2] It is taken by mouth.[1]
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Rifater, Tebrazid, others[1] |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682402 |
| License data |
|
| Routes of administration | By mouth |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | >90% |
| Metabolism | liver |
| Elimination half-life | 9 to 10 hours |
| Excretion | kidney |
| Identifiers | |
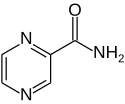
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| NIAID ChemDB | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.002.470 |
| Chemical and physical data | |
| Formula | C5H5N3O |
| Molar mass | 123.115 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Common side effects include nausea, loss of appetite, muscle and joint pains, and rash.[2][4] More serious side effects include gout, liver toxicity, and sensitivity to sunlight.[2] It is not recommended in those with significant liver disease or porphyria.[3] It is unclear if use during pregnancy is safe but it is likely okay during breastfeeding.[3] Pyrazinamide is in the antimycobacterial class of medications.[2] How it works is not entirely clear.[2]
Pyrazinamide was first made in 1936, but did not come into wide use until 1972.[5] It is on the World Health Organization's List of Essential Medicines.[6] Pyrazinamide is available as a generic medication.[2]
Medical uses
Pyrazinamide is only used in combination with other drugs such as isoniazid and rifampicin in the treatment of Mycobacterium tuberculosis and as directly observed therapy (DOT).[4] It is never used on its own. It has no other indicated medical uses. In particular, it is not used to treat other mycobacteria; Mycobacterium bovis and Mycobacterium leprae are innately resistant to pyrazinamide.
Pyrazinamide is used in the first 2 months of treatment to reduce the duration of treatment required.[7] Regimens not containing pyrazinamide must be taken for 9 months or more.
Pyrazinamide is a potent antiuricosuric drug[8] and consequently has an off-label use in the diagnosis of causes of hypouricemia and hyperuricosuria.[9] It acts on URAT1.[9]
Adverse effects
The most common (roughly 1%) side effect of pyrazinamide is joint pains (arthralgia), but this is not usually so severe that patients need to stop taking it.[10][11] Pyrazinamide can precipitate gout flares by decreasing renal excretion of uric acid.[12]
The most dangerous side effect of pyrazinamide is hepatotoxicity, which is dose-related. The old dose for pyrazinamide was 40–70 mg/kg daily and the incidence of drug-induced hepatitis has fallen significantly since the recommended dose has been reduced to 12–30 mg/kg daily. In the standard four-drug regimen (isoniazid, rifampicin, pyrazinamide, ethambutol), pyrazinamide is the most common cause of drug-induced hepatitis.[13] It is not possible to clinically distinguish pyrazinamide-induced hepatitis from hepatitis caused by isoniazid or rifampicin; test dosing is required (this is discussed in detail in tuberculosis treatment)
Other side effects include nausea and vomiting, anorexia, sideroblastic anemia, skin rash, urticaria, pruritus, dysuria, interstitial nephritis, malaise, rarely porphyria, and fever.
Pharmacokinetics
Pyrazinamide is well absorbed orally. It crosses inflamed meninges and is an essential part of the treatment of tuberculous meningitis. It is metabolised by the liver and the metabolic products are excreted by the kidneys.
Pyrazinamide is routinely used in pregnancy in the UK and the rest of the world; the World Health Organization (WHO) recommends its use in pregnancy; and extensive clinical experience shows that it is safe. In the US, pyrazinamide is not used in pregnancy, citing insufficient evidence of safety.[14] Pyrazinamide is removed by haemodialysis, so doses should always be given at the end of a dialysis session.
Mechanism of action
Pyrazinamide is a prodrug that stops the growth of M. tuberculosis.
Pyrazinamide diffuses into the granuloma of M. tuberculosis, where the tuberculosis enzyme pyrazinamidase converts pyrazinamide to the active form pyrazinoic acid.[15] Under acidic conditions of pH 5 to 6, the pyrazinoic acid that slowly leaks out converts to the protonated conjugate acid, which is thought to diffuse easily back into the bacilli and accumulate. The net effect is that more pyrazinoic acid accumulates inside the bacillus at acid pH than at neutral pH.[15][16]
Pyrazinoic acid was thought to inhibit the enzyme fatty acid synthase (FAS) I, which is required by the bacterium to synthesize fatty acids[17] although this has been discounted.[18][19] The accumulation of pyrazinoic acid was also suggested to disrupt membrane potential and interfere with energy production, necessary for survival of M. tuberculosis at an acidic site of infection. However, since an acidic environment is not essential for pyrazinamide susceptibility and pyrazinamide treatment does not lead to intrabacterial acidification nor rapid disruption of membrane potential, this model has also been discounted.[20] Pyrazinoic acid was proposed to bind to the ribosomal protein S1 (RpsA) and inhibit trans-translation,[21] but more detailed experiments have shown that it does not have this activity.[22]
The current hypothesis is that pyrazinoic acid blocks synthesis of coenzyme A. Pyrazinoic acid binds weakly to aspartate decarboxylase (PanD), triggering its degradation.[23] This is an unusual mechanism of action in that pyrazinamide does not directly block the action of its target, but indirectly triggers its destruction.
Resistance
Mutations in the pncA gene of M. tuberculosis, which encodes a pyrazinamidase and converts pyrazinamide to its active form pyrazinoic acid, are responsible for the majority of pyrazinamide resistance in M. tuberculosis strains.[24] A few pyrazinamide-resistant strains with mutations in the rpsA gene have also been identified.[21] However, a direct association between these rpsA mutations and pyrazinamide resistance has not been established. The pyrazinamide-resistant M. tuberculosis strain DHMH444, which harbors a mutation in the carboxy terminal coding region of rpsA, is fully susceptible to pyrazinoic acid and pyrazinamide resistance of this strain was previously associated with decreased pyrazinamidase activity.[25] Further, this strain was found to be susceptible to pyrazinamide in a mouse model of tuberculosis.[26] Thus, current data indicate that rpsA mutations are not likely to be associated with pyrazinamide resistance. Currently, three main methods of testing are used for pyrazinamide resistance: 1) phenotypic tests where a tuberculosis strain is grown in the presence of increasing concentrations of pyrazinamide, 2) measuring levels of pyrazinamidase enzyme produced by the tuberculosis strain, or 3) looking for mutations in the pncA gene of tuberculosis.[15] Concerns exist that the most widely used method for phenotypic resistance testing may overestimate the number of resistant strains.[27][28]
Global resistance of tuberculosis to pyrazinamide has been estimated to be in 16% of all cases, and 60% of people with multidrug-resistant tuberculosis.[15]
Abbreviations
The abbreviations PZA and Z are standard, and used commonly in the medical literature, although best practice discourages the abbreviating of drug names to prevent mistakes.
Presentation
Pyrazinamide is a generic drug, and is available in a wide variety of presentations. Pyrazinamide tablets form the bulkiest part of the standard tuberculosis treatment regimen. Pyrazinamide tablets are so large, some people find them impossible to swallow: pyrazinamide syrup is an option.
Pyrazinamide is also available as part of fixed-dose combinations with other TB drugs such as isoniazid and rifampicin (Rifater is an example).
History
Pyrazinamide was first discovered and patented in 1936, but not used against tuberculosis until 1952.[19] Its discovery as an antitubercular agent was remarkable since it has no activity against tuberculosis in vitro, due to not being active at a neutral pH, so would ordinarily not be expected to work in vivo.[29] However, nicotinamide was known to have activity against tuberculosis and pyrazinamide was thought to have a similar effect. Experiments in mice at Lederle and Merck confirmed its ability to kill tuberculosis and it was rapidly used in humans.[29]
References
- Hamilton R (2015). Tarascon Pocket Pharmacopoeia 2015 Deluxe Lab-Coat Edition. Jones & Bartlett Learning. p. 415. ISBN 9781284057560.
- "Pyrazinamide". The American Society of Health-System Pharmacists. Archived from the original on 20 December 2016. Retrieved 8 December 2016.
- World Health Organization (2009). Stuart MC, Kouimtzi M, Hill SR (eds.). WHO Model Formulary. World Health Organization. pp. 136, 140, 594, 608. hdl:10665/44053. ISBN 978-9241547659.
- Lewis, Sharon Mantik; Dirksen, Shannon Ruff; Heitkemper, Margaret M.; Bucher, Linda; Harding, Mariann (5 December 2013). Medical-surgical nursing : assessment and management of clinical problems (9th ed.). St. Louis, MO. ISBN 978-0-323-10089-2. OCLC 228373703.
- Donald, P. R.; van Helden, P. D. (2011). Antituberculosis Chemotherapy. Karger Medical and Scientific Publishers. p. 8. ISBN 978-3805596282. Archived from the original on 10 September 2017.
- World Health Organization (2019). World Health Organization model list of essential medicines: 21st list. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- "Controlled trial of four thrice-weekly regimens and a daily regimen all given for 6 months for pulmonary tuberculosis". Lancet. 1 (8213): 171–74. 1981. doi:10.1016/S0140-6736(02)95623-0. PMID 6109855.
- Spaia S, Magoula I, Tsapas G, Vayonas G (2000). "Effect of pyrazinamide and probenecid on peritoneal urate transport kinetics during continuous ambulatory peritoneal dialysis". Perit Dial Int. 20 (1): 47–52. doi:10.1177/089686080002000109. PMID 10716583. S2CID 19352495.
- Ichida K, Hosoyamada M, Hisatome I, Enomoto A, Hikita M, Endou H, Hosoya T (January 2004). "Clinical and molecular analysis of patients with renal hypouricemia in Japan-influence of URAT1 gene on urinary urate excretion". J. Am. Soc. Nephrol. 15 (1): 164–73. doi:10.1097/01.ASN.0000105320.04395.D0. PMID 14694169.
- "Controlled clinical trial of 4 short-course regimens of chemotherapy (three 6-month and one 9-month) for pulmonary tuberculosis". Tubercle. 64 (3): 153–66. 1983. doi:10.1016/0041-3879(83)90011-9. PMID 6356538.
- British Thoracic Society (1984). "A controlled trial of 6 months chemotherapy in pulmonary tuberculosis, final report: results during the 36 months after the end of chemotherapy and beyond". Br J Dis Chest. 78 (4): 330–36. doi:10.1016/0007-0971(84)90165-7. PMID 6386028.
- American Thoracic Society, CDC, Infectious Diseases Society of America (2003). "Treatment of tuberculosis" (PDF). MMWR Recomm Rep. 52 (RR-11): 1–77. PMID 12836625.
- Yee D, Valiquette C, Pelletier M, Parisien I, Rocher I, Menzies D (2003). "Incidence of serious side effects from first-line antituberculosis drugs among patients treated for active tuberculosis". Am J Respir Crit Care Med. 167 (11): 1472–77. doi:10.1164/rccm.200206-626OC. PMID 12569078.
- American Thoracic Society; Centers for Disease Control; Infectious Diseases Society of America (2003). "Treatment of Tuberculosis". Am J Respir Crit Care Med. 167 (4): 602–62. doi:10.1164/rccm.167.4.603. PMID 12588714.
- Whitfield, Michael G.; Soeters, Heidi M.; Warren, Robin M.; York, Talita; Sampson, Samantha L.; Streicher, Elizabeth M.; Helden, Paul D. van; Rie, Annelies van (28 July 2015). "A Global Perspective on Pyrazinamide Resistance: Systematic Review and Meta-Analysis". PLOS ONE. 10 (7): e0133869. Bibcode:2015PLoSO..1033869W. doi:10.1371/journal.pone.0133869. ISSN 1932-6203. PMC 4517823. PMID 26218737.
- Zhang Y, Mitchison D (January 2003). "The curious characteristics of pyrazinamide: a review". Int. J. Tuberc. Lung Dis. 7 (1): 6–21. PMID 12701830.
- Zimhony O, Cox JS, Welch JT, Vilchèze C, Jacobs WR (2000). "Pyrazinamide inhibits the eukaryotic-like fatty acid synthetase I (FASI) of Mycobacterium tuberculosis". Nature Medicine. 6 (9): 1043–47. doi:10.1038/79558. PMID 10973326. S2CID 7409751.
- Boshoff HI, Mizrahi V, Barry CE (2002). "Effects of Pyrazinamide on Fatty Acid Synthesis by Whole Mycobacterial Cells and Purified Fatty Acid Synthase I". Journal of Bacteriology. 184 (8): 2167–72. doi:10.1128/JB.184.8.2167-2172.2002. PMC 134955. PMID 11914348.
- Zhang, Ying; Mitchison, Denis; Shi, Wanliang; Zhang, Wenhong (2014). "Mechanisms of Pyrazinamide Action and Resistance". Microbiology Spectrum. 2 (4): 1–12. doi:10.1128/microbiolspec.mgm2-0023-2013. PMC 4268777. PMID 25530919.
- Peterson, Nicholas D.; Rosen, Brandon R.; Dillon, Nicholas A.; Baughn, Anthony D. (2015). "Uncoupling Environmental pH and Intrabacterial Acidification from Pyrazinamide Susceptibility in Mycobacterium tuberculosis". Antimicrobial Agents and Chemotherapy. 59 (12): 7320–26. doi:10.1128/aac.00967-15. PMC 4649215. PMID 26369957.
- Shi W, Zhang X, Jiang X, Yuan H, Lee JS, Barry CE, et al. (2011). "Pyrazinamide inhibits trans-translation in Mycobacterium tuberculosis". Science. 333 (6049): 1630–32. Bibcode:2011Sci...333.1630S. doi:10.1126/science.1208813. PMC 3502614. PMID 21835980.
- Dillon, Nicholas A.; Peterson, Nicholas D.; Feaga, Heather A.; Keiler, Kenneth C.; Baughn, Anthony D. (21 July 2017). "Anti-tubercular Activity of Pyrazinamide is Independent of trans-Translation and RpsA". Scientific Reports. 7 (1): 6135. Bibcode:2017NatSR...7.6135D. doi:10.1038/s41598-017-06415-5. ISSN 2045-2322. PMC 5522395. PMID 28733601.
- Gopal P, Sarathy JP, Yee M, et al. (2020). "Pyrazinamide triggers degradation of its target aspartate decarboxylase". Nature Communications. 11 (1): 1661. Bibcode:2020NatCo..11.1661G. doi:10.1038/s41467-020-15516-1. PMC 7125159. PMID 32245967.
- Scorpio A, Zhang Y (1996). "Mutations in pncA, a gene encoding pyrazinamidase/nicotinamidase, cause resistance to the antituberculous drug pyrazinamide in tubercle bacillus". Nature Medicine. 2 (6): 662–67. doi:10.1038/nm0696-662. PMID 8640557. S2CID 8579133.
- Speirs, R.J.; Welch, J.T.; Cynamon, M.H. (1995). "Activity of n-propyl pyrazinoate against pyrazinamide-resistant Mycobacterium tuberculosis: investigations into mechanism of action of and mechanism of resistance to pyrazinamide". Antimicrobial Agents and Chemotherapy. 39 (6): 1269–71. doi:10.1128/aac.39.6.1269. PMC 162725. PMID 7574514.
- Klemens, S.P.; Sharpe, C.A.; Cynamon, M.H. (1996). "Activity of pyrazinamide in a murine model against Mycobacterium tuberculosis isolates with various levels of in vitro susceptibility". Antimicrobial Agents and Chemotherapy. 40 (1): 14–16. doi:10.1128/AAC.40.1.14. PMC 163048. PMID 8787871.
- Chedore, Pamela; Bertucci, Lina; Wolfe, Joyce; Sharma, Meenu; Jamieson, Frances (1 January 2010). "Potential for Erroneous Results Indicating Resistance When Using the Bactec MGIT 960 System for Testing Susceptibility of Mycobacterium tuberculosis to Pyrazinamide". Journal of Clinical Microbiology. 48 (1): 300–01. doi:10.1128/JCM.01775-09. ISSN 0095-1137. PMC 2812260. PMID 19923479.
- Piersimoni, C.; Mustazzolu, A.; Iacobino, A.; Giannoni, F.; Santoro, G.; Gherardi, G.; Del Giudice, A.; Perna, R.; Fattorini, L. (1 December 2016). "Pyrazinamide susceptibility testing: proposed new standard with the BACTECTM MGITTM 960 system". The International Journal of Tuberculosis and Lung Disease. 20 (12): 1677–80. doi:10.5588/ijtld.16.0360. PMID 27931346.
- Zhang, Y.; Mitchison, D. (1 January 2003). "The curious characteristics of pyrazinamide: a review". The International Journal of Tuberculosis and Lung Disease. 7 (1): 6–21. ISSN 1027-3719. PMID 12701830.
External links
- "Pyrazinamide". Drug Information Portal. U.S. National Library of Medicine.