Reinforcement (speciation)
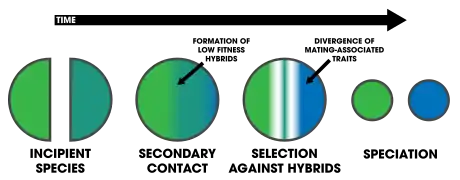
Reinforcement is a process of speciation where natural selection increases the reproductive isolation (further divided to pre-zygotic isolation and post-zygotic isolation) between two populations of species. This occurs as a result of selection acting against the production of hybrid individuals of low fitness. The idea was originally developed by Alfred Russel Wallace and is sometimes referred to as the Wallace effect. The modern concept of reinforcement originates from Theodosius Dobzhansky. He envisioned a species separated allopatrically, where during secondary contact the two populations mate, producing hybrids with lower fitness. Natural selection results from the hybrid's inability to produce viable offspring; thus members of one species who do not mate with members of the other have greater reproductive success. This favors the evolution of greater prezygotic isolation (differences in behavior or biology that inhibit formation of hybrid zygotes). Reinforcement is one of the few cases in which selection can favor an increase in prezygotic isolation, influencing the process of speciation directly.[1] This aspect has been particularly appealing among evolutionary biologists.[2]
| Part of a series on |
| Evolutionary biology |
|---|
 |
|
The support for reinforcement has fluctuated since its inception, and terminological confusion and differences in usage over history have led to multiple meanings and complications. Various objections have been raised by evolutionary biologists as to the plausibility of its occurrence. Since the 1990s, data from theory, experiments, and nature have overcome many of the past objections, rendering reinforcement widely accepted,[3]: 354 though its prevalence in nature remains unknown.[4][5]
Numerous models have been developed to understand its operation in nature, most relying on several facets: genetics, population structures, influences of selection, and mating behaviors. Empirical support for reinforcement exists, both in the laboratory and in nature. Documented examples are found in a wide range of organisms: both vertebrates and invertebrates, fungi, and plants. The secondary contact of originally separated incipient species (the initial stage of speciation) is increasing due to human activities such as the introduction of invasive species or the modification of natural habitats.[6] This has implications for measures of biodiversity and may become more relevant in the future.[6]
History
Reinforcement has had a complex history in that its popularity among scholars has changed over time.[7][8] Jerry Coyne and H. Allen Orr contend that the theory of reinforcement went through three phases of historical development:[3]: 366
- plausibility based on unfit hybrids
- implausibility based on hybrids having some fitness
- plausibility based on empirical studies and biologically complex and realistic models

Sometimes called the Wallace effect, reinforcement was originally proposed by Alfred Russel Wallace in 1889.[9]: 353 His hypothesis differed markedly from the modern conception in that it focused on post-zygotic isolation, strengthened by group selection.[10][11][3]: 353 Theodosius Dobzhansky was the first to provide a thorough description of the process in 1937,[3]: 353 though the term itself was not coined until 1955 by W. Frank Blair.[12] In 1930, Ronald Fisher laid out the first genetic description of the process of reinforcement in The Genetical Theory of Natural Selection, and in 1965 and 1970 the first computer simulations were run to test for its plausibility.[3]: 367 Later population genetic[13] and quantitative genetic[14] studies were conducted showing that completely unfit hybrids lead unequivocally to an increase in prezygotic isolation.[3]: 367
Dobzhansky's idea gained significant support; he suggested that it illustrated the final step in speciation, for example after an allopatric population comes into secondary contact.[3]: 353 In the 1980s, many evolutionary biologists began to doubt the plausibility of the idea,[3]: 353 based not on empirical evidence, but largely on the growth of theory that deemed it an unlikely mechanism of reproductive isolation.[2] A number of theoretical objections arose at the time and are addressed in the Arguments against reinforcement section below.
By the early 1990s, reinforcement saw a revival in popularity among evolutionary biologists; due primarily from a sudden increase in data—empirical evidence from studies in labs and largely by examples found in nature.[3]: 354 Further, computer simulations of the genetics and migration patterns of populations found, "something looking like reinforcement".[3]: 372 The most recent theoretical work on speciation has come from several studies (notably from Liou and Price, Kelly and Noor, and Kirkpatrick and Servedio) using highly complex computer simulations; all of which came to similar conclusions: that reinforcement is plausible under several conditions, and in many cases, is easier than previously thought.[3]: 374
Terminology
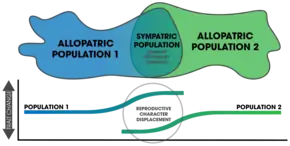
Confusion exists around the meaning of the term reinforcement.[15] It was first used to describe the observed mating call differences in Gastrophryne frogs within a secondary contact hybrid zone.[15] The term secondary contact has also been used to describe reinforcement in the context of an allopatrically separated population experiencing contact after the loss of a geographic barrier.[16] The Wallace effect is similar to reinforcement, but is rarely used.[15] Roger Butlin demarcated incomplete post-zygotic isolation from complete isolation, referring to incomplete isolation as reinforcement and completely isolated populations as experiencing reproductive character displacement.[17] Daniel J. Howard considered reproductive character displacement to represent either assortive mating or the divergence of traits for mate recognition (specifically between sympatric populations).[15] Reinforcement, under his definition, included prezygotic divergence and complete post-zygotic isolation.[18] Servedio and Noor include any detected increase in prezygotic isolation as reinforcement, as long as it is a response to selection against mating between two different species.[4] Coyne and Orr contend that, "true reinforcement is restricted to cases in which isolation is enhanced between taxa that can still exchange genes".[3]: 352
Models
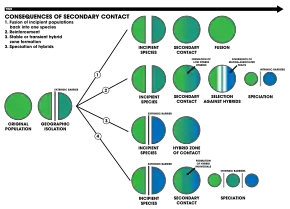
1. An extrinsic barrier separates a species population into two but they come into contact before reproductive isolation is sufficient to result in speciation. The two populations fuse back into one species
2. Speciation by reinforcement
3. Two separated populations stay genetically distinct while hybrid swarms form in the zone of contact
4. Genome recombination results in speciation of the two populations, with an additional hybrid species. All three species are separated by intrinsic reproductive barriers[19]
One of the strongest forms of reproductive isolation in nature is sexual isolation: traits in organisms involving mating.[20] This pattern has led to the idea that, because selection acts so strongly on mating traits, it may be involved in the process of speciation.[20] This process of speciation influenced by natural selection is reinforcement, and can happen under any mode of speciation[3]: 355 (e.g. geographic modes of speciation or ecological speciation[21]). It necessitates two forces of evolution that act on mate choice: natural selection and gene flow.[22] Selection acts as the main driver of reinforcement as it selects against hybrid genotypes that are of low-fitness, regardless if individual preferences have no effect on survival and reproduction.[22] Gene flow acts as the primary opposing force against reinforcement, as the exchange of genes between individuals leading to hybrids cause the genotypes to homogenize.[22]
Butlin laid out four primary criteria for reinforcement to be detected in natural or laboratory populations:[17]
- Gene flow between two taxa exists or can be established to have existed at some point.
- There is divergence of mating-associated traits between two taxa.
- Patterns of mating are modified, limiting the production of low fitness hybrids.
- Other selection pressures leading to divergence of the mate-recognition system have not occurred.
After speciation by reinforcement occurs, changes after complete reproductive isolation (and further isolation thereafter) are a form of reproductive character displacement.[23] A common signature of reinforcement's occurrence in nature is that of reproductive character displacement; characteristics of a population diverge in sympatry but not allopatry.[6][5] One difficulty in detection is that ecological character displacement can result in the same patterns.[24] Further, gene flow can diminish the isolation found in sympatric populations.[24] Two important factors in the outcome of the process rely on: 1) the specific mechanisms that causes prezygotic isolation, and 2) the number of alleles altered by mutations affecting mate choice.[25]
In instances of peripatric speciation, reinforcement is unlikely to complete speciation in the case that the peripherally isolated population comes into secondary contact with the main population.[26] In sympatric speciation, selection against hybrids is required; therefore reinforcement can play a role, given the evolution of some form of fitness trade-offs.[1] In sympatry, patterns of strong mating discrimination are often observed—being attributed to reinforcement.[7] Reinforcement is thought to be the agent of gametic isolation.[27]
Genetics
The underlying genetics of reinforcement can be understood by an ideal model of two haploid populations experiencing an increase in linkage disequilibrium. Here, selection rejects low fitness or allele combinations while favoring combinations of alleles (in the first subpopulation) and alleles (in the second subpopulation). The third locus or (the assortive mating alleles) have an effect on mating pattern but is not under direct selection. If selection at and cause changes in the frequency of allele , assortive mating is promoted, resulting in reinforcement. Both selection and assortive mating are necessary, that is, that matings of and are more common than matings of and .[28] A restriction of migration between populations can further increase the chance of reinforcement, as it decreases the probability of the differing genotypes to exchange.[15]
An alternative model exists to address the antagonism of recombination, as it can reduce the association between the alleles that involve fitness and the assortive mating alleles that do not.[15] Genetic models often differ in terms of the number of traits associated with loci;[29] with some relying on one locus per trait[26][30][31] and others on polygenic traits.[23][22][32]
Population structures
The structure and migration patterns of a population can affect the process of speciation by reinforcement. It has been shown to occur under an island model, harboring conditions with infrequent migrations occurring in one direction,[22] and in symmetric migration models where species migrate evenly back and forth between populations.[26][30]
.png.webp)
Reinforcement can also occur in single populations,[29][23] mosaic hybrid zones (patchy distributions of parental forms and subpopulations),[31] and in parapatric populations with narrow contact zones.[33]
Population densities are an important factor in reinforcement, often in conjunction with extinction.[23] It is possible that, when two species come into secondary contact, one population can become extinct—primarily due to low hybrid fitness accompanied by high population growth rates.[23] Extinction is less likely if the hybrids are inviable instead of infertile, as fertile individuals can still survive long enough to reproduce.[23]
Selection
Speciation by reinforcement relies directly on selection to favor an increase in prezygotic isolation,[1] and the nature of selection's role in reinforcement has been widely discussed, with models applying varying approaches.[29] Selection acting on hybrids can occur in several different ways. All hybrids produced may be equality low-fitness,[23] conferring a broad disadvantage. In other cases, selection may favor multiple and varying phenotypes[26] such as in the case of a mosaic hybrid zone.[31] Natural selection can act on specific alleles both directly or indirectly.[29][22][34] In direct selection, the frequency of the selected allele is favored to the extreme. In cases where an allele is indirectly selected, its frequency increases due to a different linked allele experiencing selection (linkage disequilibrium).[15]
The condition of the hybrids under selection can play a role in post-zygotic isolation, as hybrid inviability (a hybrid unable to mature into a fit adult) and sterility (the inability to produce offspring entirely) prohibit gene flow between populations.[7] Selection against the hybrids can even be driven by any failure to obtain a mate, as it is effectively indistinguishable from sterility—each circumstance results in no offspring.[7]
Mating and mate preference
Some initial divergence in mate preference must be present for reinforcement to occur.[7][23][35] Any traits that promote isolation may be subjected to reinforcement such as mating signals (e.g. courtship display), signal responses, the location of breeding grounds, the timing of mating (e.g. seasonal breeding such as in allochronic speciation), or even egg receptivity.[15] Individuals may also discriminate against mates that differ in various traits such as mating call or morphology.[36] Many of these examples are described below.
Evidence
The evidence for reinforcement comes from observations in nature, comparative studies, and laboratory experiments.[3]: 354
Nature
Reinforcement can be shown to be occurring (or to have occurred in the past) by measuring the strength of prezygotic isolation in a sympatric population in comparison to an allopatric population of the same species.[3]: 357 Comparative studies of this allow for determining large-scale patterns in nature across various taxa.[3]: 362 Mating patterns in hybrid zones can also be used to detect reinforcement.[18] Reproductive character displacement is seen as a result of reinforcement,[7] so many of the cases in nature express this pattern in sympatry. Reinforcement's ubiquity is unknown,[4] but the patterns of reproductive character displacement are found across numerous taxa and is considered to be a common occurrence in nature.[18] Studies of reinforcement in nature often prove difficult, as alternative explanations for the detected patterns can be asserted.[3]: 358 Nevertheless, empirical evidence exists for reinforcement occurring across various taxa[7] and its role in precipitating speciation is conclusive.[15]
Comparative studies
.png.webp)
Assortive mating is expected to increase among sympatric populations experiencing reinforcement.[15] This fact allows for the direct comparison of the strength of prezygotic isolation in sympatry and allopatry between different experiments and studies.[3]: 362 Coyne and Orr surveyed 171 species pairs, collecting data on their geographic mode, genetic distance, and strength of both prezygotic and postzygotic isolation; finding that prezygotic isolation was significantly stronger in sympatric pairs, correlating with the ages of the species.[3]: 362 Additionally, the strength of post-zygotic isolation was not different between sympatric and allopatric pairs.[15] This finding supports the predictions of speciation by reinforcement and correlates well with a later study[18] that found 33 studies expressing patterns of strong prezygotic isolation in sympatry.[3]: 363 A survey of the rates of speciation in fish and their associated hybrid zones found similar patterns in sympatry, supporting the occurrence of reinforcement.[38]
Laboratory experiments
Laboratory studies that explicitly test for reinforcement are limited,[3]: 357 with many of the experiments having been conducted on Drosophila fruit flies. In general, two types of experiments have been conducted: using artificial selection to mimic natural selection that eliminates the hybrids (often called "destroy-the-hybrids"), and using disruptive selection to select for a trait (regardless of its function in sexual reproduction).[3]: 355–357 Many experiments using the destroy-the-hybrids technique are generally cited as supportive of reinforcement; however, some researchers such as Coyne and Orr and William R. Rice and Ellen E. Hostert contend that they do not truly model reinforcement, as gene flow is completely restricted between two populations.[39][3]: 356
Alternative hypotheses
Various alternative explanations for the patterns observed in nature have been proposed.[3]: 375 There is no single, overarching signature of reinforcement; however, there are two proposed possibilities:[3]: 379 that of sex asymmetry (where females in sympatric populations are forced to become choosy in the face of two differing males)[40] and that of allelic dominance: any of the alleles experiencing selection for isolation should be dominate.[7] Though this signature does not fully account for fixation probabilities or ecological character displacement.[3]: 380 Coyne and Orr extend the sex asymmetry signature and contend that, regardless of the change seen in females and males in sympatry, isolation is driven more by females.[3]: 380
Ecological or ethological influences
Ecology can also play a role in the observed patterns—called ecological character displacement. Natural selection may drive the reduction of an overlap of niches between species instead of acting to reduce hybridization[3]: 377 Though one experiment in stickleback fish that explicitly tested this hypotheses found no evidence.[41]
Species interactions can also result in reproductive character displacement (in both mate preference or mating signal).[20] Examples include predation and competition pressures, parasites, deceptive pollination, and mimicry.[20] Because these and other factors can result in reproductive character displacement, Conrad J. Hoskin and Megan Higgie give five criteria for reinforcement to be distinguished between ecological and ethological influences:
(1) mating traits are identified in the focal species; (2) mating traits are affected by a species interaction, such that selection on mating traits is likely; (3) species interactions differ among populations (present vs. absent, or different species interactions affecting mating traits in each population); (4) mating traits (signal and/or preference) differ among populations due to differences in species interactions; (5) speciation requires showing that mating trait divergence results in complete or near complete sexual isolation among populations. Results will be most informative in a well-resolved biogeographic setting where the relationship and history among populations is known.[20]
Fusion
It is possible that the pattern of enhanced isolation could simply be a temporary outcome of secondary contact where two allopatric species already have a varying range of prezygotic isolation: with some exhibiting more than others.[42] Those that have weaker prezygotic isolation will eventually fuse, losing their distinctiveness.[7] This hypothesis does not explain the fact that individual species in allopatry, experiencing consistent gene flow, would not differ in levels of gene flow upon secondary contact.[7][43] Furthermore, patterns detected in Drosophila find high levels of prezygotic isolation in sympatry but not in allopatry.[44] The fusion hypothesis predicts that strong isolation should be found in both allopatry and sympatry.[44] This fusion process is thought to occur in nature, but does not fully explain the patterns found with reinforcement.[3]: 376
Sympatry
.png.webp)
It is possible that the process of sympatric speciation itself may result in the observed patterns of reinforcement.[3]: 378 One method of distinguishing between the two is to construct a phylogenetic history of the species, as the strength of prezygotic isolation between a group of related species should differ according to how they speciated in the past.[45] Two other ways to determine if reinforcement occurs (as opposed to sympatric speciation) are:
- if two recently speciated taxa do not show signs of post-zygotic isolation of both sympatric and allopatric populations (in sympatric speciation, post-zygotic isolation is not a prerequisite);[46]
- if a cline exists between two species over a range of traits (sympatric speciation does not require a cline to exist at all).[47]
Sexual selection
In a runaway process (not unlike Fisherian runaway selection), selection against the low-fitness hybrids favors assortive mating, increasing mate discrimination rapidly.[7][44] Additionally, when there is a low cost to female mate preferences, changes in male phenotypes can result, expressing a pattern identical to that of reproductive character displacement.[48] Post-zygotic isolation is not needed, initiated simply by the fact that unfit hybrids cannot get mates.[7]
Arguments against reinforcement
A number of objections were put forth, mainly during the 1980s, arguing that reinforcement is implausible.[7][20][3]: 369 Most rely on theoretical work which suggested that the antagonism between the forces of natural selection and gene flow were the largest barriers to its feasibility.[3]: 369–372 These objections have since been largely contradicted by evidence from nature.[18][3]: 372
Gene flow
Concerns about hybrid fitness playing a role in reinforcement has led to objections based on the relationship between selection and recombination.[5][3]: 369 That is, if gene flow is not zero (if hybrids aren't completely unfit), selection cannot drive the fixation of alleles for prezygotic isolation.[28] For example: If population has the prezygotic isolating allele and the high fitness, post-zygotic alleles and ; and population has the prezygotic allele a and the high fitness, post-zygotic alleles and , both and genotypes will experience recombination in the face of gene flow. Somehow, the populations must be maintained.[3]: 369
In addition, specific alleles that have the selective advantage within the overlapped populations are only useful within that population.[49] However, if they are selectively advantageous, gene flow should allow the alleles to spread throughout both populations.[49] To prevent this, the alleles would have to be deleterious or neutral.[3]: 371 This is not without problems, as gene flow from the presumably large allopatric regions could overwhelm the area when two populations overlap.[3]: 371 For reinforcement to work, gene flow must be present, but very limited.[26][31]
Recent studies suggest reinforcement can occur under a wider range of conditions than previously thought[29][46][3]: 372–373 and that the effect of gene flow can be overcome by selection.[50][51] For example, the two species Drosophila santomea and D. yakuba on the African island São Tomé occasionally hybridize with one another, resulting in fertile female offspring and sterile male offspring.[50] This natural setting was reproduced in the laboratory, directly modeling reinforcement: the removal of some hybrids and the allowance of varying levels of gene flow.[51] The results of the experiment strongly suggested that reinforcement works under a variety of conditions, with the evolution of sexual isolation arising in 5–10 fruit fly generations.[51]
Rapid requirements
In conjunction with the fusion hypothesis, reinforcement can be thought of as a race against both fusion and extinction.[42] The production of unfit hybrids is effectively the same as a heterozygote disadvantage; whereby a deviation from genetic equilibrium causes the loss of the unfit allele.[52] This effect would result in the extinction of one of the populations.[53] This objection is overcome by when both populations are not subject to the same ecological conditions.[3]: 370 Though, it is still possible for extinction of one population to occur, and has been shown in population simulations.[54] For reinforcement to occur, prezygotic isolation must happen quickly.[3]: 370
References
- Hannes Schuler, Glen R. Hood, Scott P. Egan, and Jeffrey L. Feder (2016), Meyers, Robert A (ed.), "Modes and Mechanisms of Speciation", Reviews in Cell Biology and Molecular Medicine, 2 (3): 60–93, doi:10.1002/3527600906, ISBN 9783527600908
{{citation}}: CS1 maint: multiple names: authors list (link) - Jeremy L. Marshall, Michael L. Arnold, and Daniel J. Howard (2002), "Reinforcement: the road not taken", Trends in Ecology & Evolution, 17 (12): 558–563, doi:10.1016/S0169-5347(02)02636-8
{{citation}}: CS1 maint: multiple names: authors list (link) - Jerry A. Coyne; H. Allen Orr (2004), Speciation, Sinauer Associates, pp. 1–545, ISBN 978-0-87893-091-3
- Maria R. Servedio; Mohamed A. F. Noor (2003), "The Role of Reinforcement in Speciation: Theory and Data", Annual Review of Ecology, Evolution, and Systematics, 34: 339–364, doi:10.1146/annurev.ecolsys.34.011802.132412
- Daniel Ortíz-Barrientos, Alicia Grealy, and Patrik Nosil (2009), "The Genetics and Ecology of Reinforcement: Implications for the Evolution of Prezygotic Isolation in Sympatry and Beyond", Annals of the New York Academy of Sciences, 1168: 156–182, doi:10.1111/j.1749-6632.2009.04919.x, PMID 19566707, S2CID 4598270
{{citation}}: CS1 maint: multiple names: authors list (link) - Maria R. Servedio (2004), "The What and Why of Research on Reinforcement", PLOS Biology, 2 (12): 2032–2035, doi:10.1371/journal.pbio.0020420, PMC 535571, PMID 15597115
- Mohamed A. F. Noor (1999), "Reinforcement and other consequences of sympatry", Heredity, 83 (5): 503–508, doi:10.1038/sj.hdy.6886320, PMID 10620021, S2CID 26625194
- Roger K. Butlin and Carole M. Smadja (2018), "Coupling, Reinforcement, and Speciation" (PDF), The American Naturalist, 191 (2): 155–172, doi:10.1086/695136, PMID 29351021, S2CID 3397377
- Wallace, Alfred Russel (1889). Darwinism. Macmillan & Co. pp. 174–179.
- M. J. Littlejohn (1981). Reproductive isolation: A critical review. In W. R. Atchley and D. S. Woodruff (eds) Evolution and Speciation, Cambridge University Press, Pp. 298–334.
- Mario A. Fares (2015), Natural Selection: Methods and Applications, CRC Press, p. 3, ISBN 9781482263725
- Blair, W. Frank (1955), "Mating call and stage of speciation in the Microhyla olivacea-M. carolinensis complex", Evolution, 9 (4): 469–480, doi:10.1111/j.1558-5646.1955.tb01556.x, S2CID 88238743
- Stanley Sawyer and Daniel Hartl (1981), "On the evolution of behavioral reproductive isolation: The Wallace effect", Theoretical Population Biology, 19 (1): 261–273, doi:10.1016/0040-5809(81)90021-6
- J. A. Sved (1981), "A Two-Sex Polygenic Model for the Evolution of Premating Isolation. I. Deterministic Theory for Natural Populations", Genetics, 97 (1): 197–215, doi:10.1093/genetics/97.1.197, PMC 1214384, PMID 17249073
- Glenn-Peter Sætre (2012). "Reinforcement". eLS. doi:10.1002/9780470015902.a0001754.pub3. ISBN 978-0470016176.
{{cite book}}: Missing or empty|title=(help) - Dobzhansky, Theodosius (1937). Genetics and the Origin of Species. Columbia University Press.
- Butlin, Roger K. (1989). "Reinforcement of premating isolation". In Otte, D.; Endler, John A. (eds.). Speciation and its Consequences. Sinauer Associates. pp. 158–179. ISBN 978-0-87893-657-1.
- Howard, Daniel J. (1993). "Reinforcement: origin, dynamics and fate of an evolutionary hypothesis". In Harrison, R. G. (ed.). Hybrid Zones and the Evolutionary Process. Oxford University Press. pp. 46–69. ISBN 978-0-19-506917-4.
- John A. Hvala and Troy E. Wood (2012). "Speciation: Introduction". eLS. doi:10.1002/9780470015902.a0001709.pub3. ISBN 978-0470016176.
{{cite book}}: Missing or empty|title=(help) - Conrad J. Hoskin and Megan Higgie (2010), "Speciation via species interactions: the divergence of mating traits within species", Ecology Letters, 13 (4): 409–420, doi:10.1111/j.1461-0248.2010.01448.x, PMID 20455922, S2CID 16175451
- Mark Kirkpatrick (2001), "Reinforcement during ecological speciation", Proceedings of the Royal Society B, 268 (1473): 1259–1263, doi:10.1098/rspb.2000.1427, PMC 1088735, PMID 11410152
- Mark Kirkpatrick and Maria R. Servedio (1999), "The reinforcement of mating preferences on an island", Genetics, 151 (2): 865–884, doi:10.1093/genetics/151.2.865, PMC 1460501, PMID 9927476
- Lily W. Liou and Trevor D. Price (1994), "Speciation by reinforcement of premating isolation", Evolution, 48 (5): 1451–1459, doi:10.1111/j.1558-5646.1994.tb02187.x, PMID 28568419, S2CID 22630822
- The Marie Curie SPECIATION Network (2012), "What do we need to know about speciation?", Trends in Ecology & Evolution, 27 (1): 27–39, doi:10.1016/j.tree.2011.09.002, PMID 21978464
- Claudia Bank, Joachim Hermission, and Mark Kirkpatrick (2012), "Can reinforcement complete speciation?", Evolution, 66 (1): 229–239, doi:10.1111/j.1558-5646.2011.01423.x, PMID 22220877, S2CID 15602575
{{citation}}: CS1 maint: multiple names: authors list (link) - Maria R. Servedio and Mark Kirkpatrick (1997), "The effects of gene flow on reinforcement", Evolution, 51 (6): 1764–1772, doi:10.1111/j.1558-5646.1997.tb05100.x, PMID 28565111, S2CID 12269299
- Daniel R. Matute (2010), "Reinforcement of Gametic Isolation in Drosophila", PLOS Biology, 8 (6): e1000341, doi:10.1371/journal.pbio.1000341, PMC 2843595, PMID 20351771
- Joseph Felsenstein (1981), "Skepticism Towards Santa Rosalia, or Why are There so Few Kinds of Animals?", Evolution, 35 (1): 124–138, doi:10.2307/2407946, JSTOR 2407946, PMID 28563447
- Michael Turelli; Nicholas H. Barton; Jerry A. Coyne (2001), "Theory and speciation", Trends in Ecology & Evolution, 16 (7): 330–343, doi:10.1016/S0169-5347(01)02177-2, PMID 11403865
- Maria R. Servedio (2000), "Reinforcement and the genetics of nonrandom mating", Evolution, 54 (1): 21–29, doi:10.1111/j.0014-3820.2000.tb00003.x, PMID 10937179, S2CID 12563023
- Michael L. Cain, Viggo Andreasen, and Daniel J. Howard (1999), "Reinforcing selection is effective under a relatively broad set of conditions in a mosaic hybrid zone", Evolution, 53 (5): 1343–1353, doi:10.1111/j.1558-5646.1999.tb05399.x, PMID 28565558, S2CID 31107731
{{citation}}: CS1 maint: multiple names: authors list (link) - Mark Kirkpatrick (2000), "Reinforcement and divergence under assortive mating", Proceedings of the Royal Society B, 267 (1453): 1649–1655, doi:10.1098/rspb.2000.1191, PMC 1690725, PMID 11467428
- Neil Sanderson (1989), "Can gene flow prevent reinforcement?", Evolution, 43 (6): 1223–1235, doi:10.2307/2409358, JSTOR 2409358, PMID 28564502
- Maria R. Servedio (2001), "Beyond reinforcement: The evolution of premating isolation by direct selection on preferences and postmating, prezygotic incompatibilities", Evolution, 55 (10): 1909–1920, doi:10.1111/j.0014-3820.2001.tb01309.x, PMID 11761053, S2CID 25296147
- J. K. Kelly and Mohamed A. F. Noor (1996), "Speciation by reinforcement: a model derived from studies of Drosophila", Genetics, 143 (3): 1485–1497, doi:10.1093/genetics/143.3.1485, PMC 1207414, PMID 8807317
- Conrad J. Hoskin, Megan Higgie, Keith R. McDonald, and Craig Moritz (2005), "Reinforcement drives rapid allopatric speciation", Nature, 437 (7063): 1353–1356, Bibcode:2005Natur.437.1353H, doi:10.1038/nature04004, PMID 16251964, S2CID 4417281
{{citation}}: CS1 maint: multiple names: authors list (link) - Jerry A. Coyne and H. Allen Orr (1997), ""Patterns of Speciation in Drosophila" Revisited", Evolution, 51 (1): 295–303, doi:10.1111/j.1558-5646.1997.tb02412.x, PMID 28568795, S2CID 40390753
- A. R. McCune and N. R. Lovejoy. (1998). The relative rate of sympatric and allopatric speciation in fishes. In D. J. Howard and S. H. Berlocher (eds) Endless Forms: Species and Speciation, Oxford University Press, pp. 172–185.
- William R. Rice and Ellen E. Hostert (1993), "Laboratory Experiments on Speciation: What Have We Learned in 40 Years?", Evolution, 47 (6): 1637–1653, doi:10.1111/j.1558-5646.1993.tb01257.x, PMID 28568007, S2CID 42100751
- L. Partridge and G. A. Parker. (1999). Sexual conflict and speciation. In A. E. Magurran and R. M. May (eds) Evolution of Biological Diversity. Oxford University Press, pp.130–159
- Howard D. Rundle (1998), "Reinforcement of stickleback mate preferences: Sympatry breeds contempt", Dolph Schluter, 52 (1): 200–208, doi:10.1111/j.1558-5646.1998.tb05153.x, hdl:2429/6366, PMID 28568163, S2CID 40648544
- Alan R. Templeton (1981), "Mechanisms of Speciation – A Population Genetic Approach", Annual Review of Ecology, Evolution, and Systematics, 12: 23–48, doi:10.1146/annurev.es.12.110181.000323
- Mohamed A. F. Noor (1995), "Speciation driven by natural-selection in Drosophila", Nature, 375 (6533): 674–675, Bibcode:1995Natur.375..674N, doi:10.1038/375674a0, PMID 7791899, S2CID 4252448
- Jerry A. Coyne; H. Allen Orr (1989), "Patterns of speciation in Drosophila", Evolution, 43 (2): 362–381, doi:10.1111/j.1558-5646.1989.tb04233.x, PMID 28568554, S2CID 1678429
- Mohamed A. F. Noor (1997), "How often does sympatry affect sexual isolation in Drosophila?", The American Naturalist, 149 (6): 1156–1163, doi:10.1086/286044, PMID 18811269, S2CID 5406442
- Mark Kirkpatrick and Virginie Ravigné (2002), "Speciation by Natural and Sexual Selection: Models and Experiments", The American Naturalist, 159: S22–35, doi:10.1086/338370, PMID 18707367, S2CID 16516804
- N. H. Barton and G. M. Hewitt (1989), "Adaptation, speciation and hybrid zones", Nature, 341 (6242): 497–503, Bibcode:1989Natur.341..497B, doi:10.1038/341497a0, PMID 2677747, S2CID 4360057
- Troy Day (2000), "Sexual Selection and the Evolution of Costly Female Preferences: Spatial Effects", Evolution, 54 (3): 715–730, doi:10.1554/0014-3820(2000)054[0715:SSATEO]2.3.CO;2, PMID 10937247, S2CID 27621958
- J. A. Moore. (1957). An embryologist's view of the species concept. In Ernst Mayr (eds) The Species Problem, American Association for the Advancement of Science, pp. 325–338.
- Daniel R. Matute (2010), "Reinforcement Can Overcome Gene Flow during Speciation in Drosophila", Current Biology, 20 (24): 2229–2233, doi:10.1016/j.cub.2010.11.036, PMC 3019097, PMID 21129972
- Jerry A. Coyne (2010), "Reinforcement" and the origin of species, Wordpress
- A. A. Harper and D. M. Lambert (1983), "The population genetics of reinforcing selection", Genetica, 62 (1): 15–23, doi:10.1007/BF00123305, S2CID 7947934
- H. E. H. Paterson (1978), "More evidence against speciation by reinforcement", South African Journal of Science, 74: 369–371
- Hamish G. Spencer, Brian H. McArdle, and David M. Lambert (1986), "A Theoretical Investigation of Speciation by Reinforcement", The American Naturalist, 128 (2): 241–262, doi:10.1086/284557, S2CID 83906561
{{citation}}: CS1 maint: multiple names: authors list (link)
