Timeline of chemical element discoveries
The discovery of the 118 chemical elements known to exist as of 2022 is presented in chronological order. The elements are listed generally in the order in which each was first defined as the pure element, as the exact date of discovery of most elements cannot be accurately determined. There are plans to synthesize more elements, and it is not known how many elements are possible.
| Part of a series on the |
| Periodic table |
|---|
|
Sets of elements |
|
Each element's name, atomic number, year of first report, name of the discoverer, and notes related to the discovery are listed.
Periodic table of elements
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group → | ||||||||||||||||||||||||||||||||||||||||
| ↓ Period | ||||||||||||||||||||||||||||||||||||||||
| 1 | 1 H |
2 He | ||||||||||||||||||||||||||||||||||||||
| 2 | 3 Li |
4 Be |
5 B |
6 C |
7 N |
8 O |
9 F |
10 Ne | ||||||||||||||||||||||||||||||||
| 3 | 11 Na |
12 Mg |
13 Al |
14 Si |
15 P |
16 S |
17 Cl |
18 Ar | ||||||||||||||||||||||||||||||||
| 4 | 19 K |
20 Ca |
21 Sc |
22 Ti |
23 V |
24 Cr |
25 Mn |
26 Fe |
27 Co |
28 Ni |
29 Cu |
30 Zn |
31 Ga |
32 Ge |
33 As |
34 Se |
35 Br |
36 Kr | ||||||||||||||||||||||
| 5 | 37 Rb |
38 Sr |
39 Y |
40 Zr |
41 Nb |
42 Mo |
43 Tc |
44 Ru |
45 Rh |
46 Pd |
47 Ag |
48 Cd |
49 In |
50 Sn |
51 Sb |
52 Te |
53 I |
54 Xe | ||||||||||||||||||||||
| 6 | 55 Cs |
56 Ba |
71 Lu |
72 Hf |
73 Ta |
74 W |
75 Re |
76 Os |
77 Ir |
78 Pt |
79 Au |
80 Hg |
81 Tl |
82 Pb |
83 Bi |
84 Po |
85 At |
86 Rn | ||||||||||||||||||||||
| 7 | 87 Fr |
88 Ra |
103 Lr |
104 Rf |
105 Db |
106 Sg |
107 Bh |
108 Hs |
109 Mt |
110 Ds |
111 Rg |
112 Cn |
113 Nh |
114 Fl |
115 Mc |
116 Lv |
117 Ts |
118 Og | ||||||||||||||||||||||
| 57 La |
58 Ce |
59 Pr |
60 Nd |
61 Pm |
62 Sm |
63 Eu |
64 Gd |
65 Tb |
66 Dy |
67 Ho |
68 Er |
69 Tm |
70 Yb |
|||||||||||||||||||||||||||
| 89 Ac |
90 Th |
91 Pa |
92 U |
93 Np |
94 Pu |
95 Am |
96 Cm |
97 Bk |
98 Cf |
99 Es |
100 Fm |
101 Md |
102 No |
|||||||||||||||||||||||||||
Primordial From decay Synthetic Border shows natural occurrence of the element
| ||||||||||||||||||||||||||||||||||||||||
Ancient discoveries
| Z | Element | Earliest use | Oldest existing sample |
Discoverer(s) | Place of oldest sample |
Notes |
|---|---|---|---|---|---|---|
| 29 | Copper | 9000 BC | 6000 BC | Middle East | Anatolia | Copper was probably the first metal mined and crafted by humans.[1] It was originally obtained as a native metal and later from the smelting of ores. Earliest estimates of the discovery of copper suggest around 9000 BC in the Middle East. It was one of the most important materials to humans throughout the Chalcolithic and Bronze Ages. Copper beads dating from 6000 BC have been found in Çatal Höyük, Anatolia[2] and the archaeological site of Belovode on the Rudnik mountain in Serbia contains the world's oldest securely dated evidence of copper smelting from 5000 BC.[3][4] |
| 82 | Lead | 7000 BC | 3800 BC | Africa | Abydos, Egypt | It is believed that lead smelting began at least 9,000 years ago, and the oldest known artifact of lead is a statuette found at the temple of Osiris on the site of Abydos dated around 3800 BC.[5] |
| 79 | Gold | Before 6000 BC | Before 4000 BC | Levant | Wadi Qana | The earliest gold artifacts were discovered at the site of Wadi Qana in the Levant.[6] |
| 47 | Silver | Before 5000 BC | ca. 4000 BC | Asia Minor | Asia Minor | Estimated to have been discovered in Asia Minor shortly after copper and gold.[7][8] |
| 26 | Iron | Before 5000 BC | 4000 BC | Middle East | Egypt | There is evidence that iron was known from before 5000 BC.[9] The oldest known iron objects used by humans are some beads of meteoric iron, made in Egypt in about 4000 BC. The discovery of smelting around 3000 BC led to the start of the Iron Age around 1200 BC[10] and the prominent use of iron for tools and weapons.[11] |
| 6 | Carbon | 3750 BC | 2500 BC | Egyptians and Sumerians | Middle East | The earliest known use of charcoal was for the reduction of copper, zinc, and tin ores in the manufacture of bronze, by the Egyptians and Sumerians.[12] Diamonds were probably known as early as 2500 BC.[13] True chemical analyses were made in the 18th century,[14] and in 1789 carbon was listed by Antoine Lavoisier as an element.[15] |
| 50 | Tin | 3500 BC | 2000 BC | Asia Minor | Kestel | First smelted in combination with copper around 3500 BC to produce bronze (and thus giving place to the Bronze Age in those places where Iron Age did not intrude directly on Neolithic of the Stone Age).[16] Kestel, in southern Turkey, is the site of an ancient Cassiterite mine that was used from 3250 to 1800 BC.[17] The oldest artifacts date from around 2000 BC.[18] |
| 16 | Sulfur | Before 2000 BC | / | Middle East | Middle East | First used at least 4,000 years ago.[19] According to the Ebers Papyrus, a sulfur ointment was used in ancient Egypt to treat granular eyelids.[20] Designated as one of the two elements of which all metals are composed in the sulfur-mercury theory of metals, first described in pseudo-Apollonius of Tyana's Sirr al-khaliqa ('Secret of Creation') and in the works attributed to Jabir ibn Hayyan (both 8th or 9th century).[21] Designated as a univeral element (one of the tria prima) by Paracelsus in the early 16th century. Recognized as an element by Antoine Lavoisier in 1777. |
| 80 | Mercury | 1500 BC | 1500 BC | Egyptians | Egypt | Found in Egyptian tombs dating from 1500 BC.[22] |
| 30 | Zinc | Before 1000 BC | 1000 BC | Indian metallurgists | Indian subcontinent | Used as a component of brass since antiquity (before 1000 BC) by Indian metallurgists, but its true nature was not understood in ancient times. Identified as a distinct metal in the Rasaratna Samuccaya around the 14th century of the Christian era[23] and by the alchemist Paracelsus in 1526.[24] Isolated by Andreas Sigismund Marggraf in 1746.[25] |
| 78 | Platinum | c. 600 BC – AD 200 | c. 600 BC – AD 200 | Pre-Columbian South Americans | South America | Used by pre-Columbian Americans near modern-day Esmeraldas, Ecuador to produce artifacts of a white gold-platinum alloy, although precise dating is difficult.[26] First European description of a metal found in South American gold was in 1557 by Julius Caesar Scaliger. Antonio de Ulloa was on an expedition to Peru in 1735, where he observed the metal; he published his findings in 1748. Sir Charles Wood also investigated the metal in 1741. First reference to it as a new metal was made by William Brownrigg in 1750.[27] |
| 33 | Arsenic | c. 850–950 | c. 850–950 | Middle-Eastern alchemists | Middle East | The use of metallic arsenic was described by the Egyptian alchemist Zosimos.[28] The purification of arsenic was later described in the works attributed to the Muslim alchemist Jabir ibn Hayyan (c. 850–950).[29] Albertus Magnus (c. 1200-1280) is typically credited with the description of the metalloid in the West.[30] |
| 51 | Antimony | c. 850–950 | c. 850–950 | Jabir ibn Hayyan | Middle East | Dioscorides and Pliny both describe the accidental production of metallic antimony from stibnite, but only seem to recognize the metal as lead.[31] The intentional isolation of antimony is described in the works attributed to the Muslim alchemist Jabir ibn Hayyan (c. 850–950).[29] In Europe, the metal was being produced and used by 1540, when it was described by Vannoccio Biringuccio.[32] |
| 83 | Bismuth | c. 1500[33] | c. 1500 | European alchemists and Inca civilisation | Europe and South America | Bismuth was known since ancient times, but often confused with tin and lead, which are chemically similar. The Incas used bismuth (along with the usual copper and tin) in a special bronze alloy for knives.[34] Agricola (1546) states that bismuth is a distinct metal in a family of metals including tin and lead. This was based on observation of the metals and their physical properties.[35] Miners in the age of alchemy also gave bismuth the name tectum argenti, or "silver being made" in the sense of silver still in the process of being formed within the Earth.[36][37][38] Beginning with Johann Heinrich Pott in 1738,[39] Carl Wilhelm Scheele, and Torbern Olof Bergman, the distinctness of lead and bismuth became clear, and Claude François Geoffroy demonstrated in 1753 that this metal is distinct from lead and tin.[37][40][41] |
Modern discoveries
| Z | Element | Observed or predicted | Isolated (widely known) | Notes | ||
|---|---|---|---|---|---|---|
| By | By | |||||
| 15 | Phosphorus | 1669 | H. Brand | 1669 | H. Brand | Prepared from urine, it was the first element whose discovery date and discoverer is recorded.[42] |
| 27 | Cobalt | 1735 | G. Brandt | 1735 | G. Brandt | Proved that the blue color of glass is due to a new kind of metal and not bismuth as thought previously.[43] |
| 28 | Nickel | 1751 | F. Cronstedt | 1751 | F. Cronstedt | Found by attempting to extract copper from the mineral known as fake copper (now known as niccolite).[44] |
| 1 | Hydrogen | 1766 | H. Cavendish | 1766 | H. Cavendish | Cavendish was the first to distinguish H 2 from other gases, although Paracelsus around 1500, Robert Boyle, and Joseph Priestley had observed its production by reacting strong acids with metals. Lavoisier named it in 1783.[45][46] It was the first elemental gas known. |
| 8 | Oxygen | 1771 | W. Scheele | 1771 | W. Scheele | Scheele obtained it by heating mercuric oxide and nitrates in 1771, but did not publish his findings until 1777. Joseph Priestley also prepared this new air by 1774, but only Lavoisier recognized it as a true element; he named it in 1777.[47][48] Before him, Sendivogius had produced oxygen by heating saltpetre, correctly identifying it as the "food of life".[49] |
| 7 | Nitrogen | 1772 | D. Rutherford | 1772 | D. Rutherford | Rutherford discovered nitrogen while studying at the University of Edinburgh.[50] He showed that the air in which animals had breathed, even after removal of the exhaled carbon dioxide, was no longer able to burn a candle. Carl Wilhelm Scheele, Henry Cavendish, and Joseph Priestley also studied the element at about the same time, and Lavoisier named it in 1775–6.[51] |
| 17 | Chlorine | 1774 | W. Scheele | 1774 | W. Scheele | Obtained it from hydrochloric acid, but thought it was an oxide. Only in 1808 did Humphry Davy recognize it as an element.[52] |
| 25 | Manganese | 1774 | W. Scheele | 1774 | G. Gahn | Distinguished pyrolusite as the calx of a new metal. Ignatius Gottfred Kaim also discovered the new metal in 1770, as did Scheele in 1774. It was isolated by reduction of manganese dioxide with carbon.[53] |
| 42 | Molybdenum | 1778 | W. Scheele | 1781 | J. Hjelm | Scheele recognised the metal as a constituent of molybdena.[54] |
| 74 | Tungsten | 1781 | W. Scheele | 1783 | J. and F. Elhuyar | Scheele obtained from scheelite an oxide of a new element. The Elhuyars obtained tungstic acid from wolframite and reduced it with charcoal.[55] |
| 52 | Tellurium | 1782 | F.-J.M. von Reichenstein | H. Klaproth | Muller observed it as an impurity in gold ores from Transylvania.[56] | |
| 1789 | A. Lavoisier | Lavoisier writes the first modern list of chemical elements – containing 33 elements including light, heat, unextracted "radicals" and some oxides.[57] He also redefines the term "element". Until then, no metals except mercury were considered elements. | ||||
| 92 | Uranium | 1789 | H. Klaproth | 1841 | E.-M. Péligot | Klaproth mistakenly identified a uranium oxide obtained from pitchblende as the element itself and named it after the recently discovered planet Uranus.[58][59] |
| 22 | Titanium | 1791 | W. Gregor | 1825 | J. Berzelius | Gregor found an oxide of a new metal in ilmenite; Klaproth independently discovered the element in rutile in 1795 and named it. The pure metallic form was only obtained in 1910 by Matthew A. Hunter.[60][61] |
| 24 | Chromium | 1797 | N. Vauquelin | 1797 | N. Vauquelin | Vauquelin discovered the trioxide in crocoite ore in 1794, and later isolated the metal by heating the oxide in a charcoal oven.[62][63] |
| 41 | Niobium | 1801 | C. Hatchett | 1864 | W. Blomstrand | Hatchett found the element in columbite ore and named it columbium. Heinrich Rose proved in 1844 that the element is distinct from tantalum, and renamed it niobium which was officially accepted in 1949.[64] |
| 73 | Tantalum | 1802 | G. Ekeberg | Ekeberg found another element in minerals similar to columbite and in 1844, Heinrich Rose proved that it was distinct from niobium.[65] | ||
| 46 | Palladium | 1802 | W. H. Wollaston | 1802 | W. H. Wollaston | Wollaston discovered it in samples of platinum from South America, but did not publish his results immediately. He had intended to name it after the newly discovered asteroid, Ceres, but by the time he published his results in 1804, cerium had taken that name. Wollaston named it after the more recently discovered asteroid Pallas.[66] |
| 58 | Cerium | 1803 | H. Klaproth, J. Berzelius, and W. Hisinger | 1838 | G. Mosander | Berzelius and Hisinger discovered the element in ceria and named it after the newly discovered asteroid (then considered a planet), Ceres. Klaproth discovered it simultaneously and independently in some tantalum samples. Mosander proved later that the samples of all three researchers had at least another element in them, lanthanum.[67] |
| 76 | Osmium | 1803 | S. Tennant | 1803 | S. Tennant | Tennant had been working on samples of South American platinum in parallel with Wollaston and discovered two new elements, which he named osmium and iridium.[68] |
| 77 | Iridium | 1803 | S. Tennant | 1803 | S. Tennant | Tennant had been working on samples of South American platinum in parallel with Wollaston and discovered two new elements, which he named osmium and iridium, and published the iridium results in 1804.[69] |
| 45 | Rhodium | 1804 | H. Wollaston | 1804 | H. Wollaston | Wollaston discovered and isolated it from crude platinum samples from South America.[70] |
| 19 | Potassium | 1807 | H. Davy | 1807 | H. Davy | Davy discovered it by using electrolysis on potash.[71] |
| 11 | Sodium | 1807 | H. Davy | 1807 | H. Davy | Andreas Sigismund Marggraf recognised the difference between soda ash and potash in 1758. Davy discovered sodium a few days after potassium, by using electrolysis on sodium hydroxide.[72] |
| 5 | Boron | 1808 | L. Gay-Lussac and L.J. Thénard | 1808 | H. Davy | Radical boracique appears on the list of elements in Lavoisier's Traité Élémentaire de Chimie from 1789.[57] On June 21, 1808, Lussac and Thénard announced a new element in sedative salt, Davy announced the isolation of a new substance from boracic acid on June 30.[73] |
| 56 | Barium | 1808 | H. Davy | 1808 | H. Davy | Scheele distinguished a new earth (BaO) in pyrolusite in 1772 and Davy isolated the metal by electrolysis.[74] |
| 38 | Strontium | 1808 | H. Davy | 1808 | H. Davy | W. Cruikshank in 1787 and Adair Crawford in 1790 concluded that strontianite contained a new earth. It was eventually isolated electrochemically in 1808 by Davy.[75] |
| 20 | Calcium | 1808 | H. Davy | 1808 | H. Davy | Davy discovered the metal by electrolysis of quicklime.[72] |
| 12 | Magnesium | 1808 | H. Davy | 1808 | H. Davy | Joseph Black observed that magnesia alba (MgO) was not quicklime (CaO) in 1755. Davy isolated the metal electrochemically from magnesia.[76] |
| 14 | Silicon | 1808 | H. Davy | 1823 | J. Berzelius | Davy thought in 1800 that silica was a compound, not an element, and in 1808 he proved this although he could not isolate the element, and suggested the present name.[77][78] In 1811 Louis-Joseph Gay-Lussac and Louis-Jacques Thénard probably prepared impure silicon,[79] and Berzelius obtained the pure element in 1823.[80] |
| 13 | Aluminium | 1808 | H. Davy | 1824 | H.C.Ørsted | Antoine Lavoisier predicted in 1787 that alumina is the oxide of an undiscovered element, and in 1808 Davy tried to decompose it. Although he failed, he proved Lavoisier correct and suggested the present name.[81][82] Hans Christian Ørsted was the first to isolate metallic aluminium in 1824.[83][84] |
| 40 | Zirconium | 1808 | H. Davy | 1824 | J. Berzelius | Martin Heinrich Klaproth identified a new oxide in zircon in 1789,[85][86] and in 1808 Davy showed that this oxide has a metallic base although he could not isolate it.[87][88] |
| 4 | Beryllium | 1808 | H. Davy | 1828 | F. Wöhler and A. Bussy | Vauquelin discovered the oxide in beryl and emerald in 1798, and in 1808 Davy showed that this oxide has a metallic base although he could not isolate it.[89][90] Klaproth suggested the present name around 1808.[91] |
| 39 | Yttrium | 1808 | H. Davy | 1843 | H. Rose | Johan Gadolin discovered the earth in gadolinite in 1794, but Mosander showed later that its ore, yttria, contained more elements.[92][93] In 1808, Davy showed that yttria is a metallic oxide, although he could not isolate the metal.[94][95] Wöhler mistakenly thought he had isolated the metal in 1828 from a volatile chloride he supposed to be yttrium chloride,[96][97] but Rose proved otherwise in 1843 and correctly isolated the element himself that year. |
| 9 | Fluorine | 1810 | A.-M. Ampère | 1886 | H. Moissan | Radical fluorique appears on the list of elements in Lavoisier's Traité Élémentaire de Chimie from 1789, but radical muriatique also appears instead of chlorine.[57] André-Marie Ampère predicted an element analogous to chlorine obtainable from hydrofluoric acid, and between 1812 and 1886 many researchers tried to obtain this element. It was eventually isolated by Moissan.[98] |
| 53 | Iodine | 1811 | B. Courtois | 1811 | B. Courtois | Courtois discovered it in the ashes of seaweed.[99] |
| 3 | Lithium | 1817 | A. Arfwedson | 1821 | W. T. Brande | Arfwedson discovered the alkali in petalite.[100] |
| 48 | Cadmium | 1817 | S. L Hermann, F. Stromeyer, and J.C.H. Roloff | 1817 | S. L Hermann, F. Stromeyer, and J.C.H. Roloff | All three found an unknown metal in a sample of zinc oxide from Silesia, but the name that Stromeyer gave became the accepted one.[101] |
| 34 | Selenium | 1817 | J. Berzelius and G. Gahn | 1817 | J. Berzelius and G. Gahn | While working with lead they discovered a substance that they thought was tellurium, but realized after more investigation that it was different.[102] |
| 35 | Bromine | 1825 | J. Balard and C. Löwig | 1825 | J. Balard and C. Löwig | They both discovered the element in the autumn of 1825. Balard published his results the next year,[103] but Löwig did not publish until 1827.[104] |
| 90 | Thorium | 1829 | J. Berzelius | 1914 | D. Lely, Jr. and L. Hamburger | Berzelius obtained the oxide of a new earth in thorite.[105] |
| 23 | Vanadium | 1830 | N. G. Sefström | 1867 | H.E.Roscoe | Andrés Manuel del Río found the metal in vanadinite in 1801, but retracted the claim after Hippolyte Victor Collet-Descotils disputed it. Nils Gabriel Sefström rediscovered the element and named it, and later it was shown that del Río had been right in the first place.[106] |
| 57 | Lanthanum | 1838 | G. Mosander | 1841 | G. Mosander | Mosander found a new element in samples of ceria and published his results in 1842, but later he showed that this lanthana contained four more elements.[107] |
| 68 | Erbium | 1843 | G. Mosander | 1879 | T. Cleve | Mosander managed to split the old yttria into yttria proper and erbia, and later terbia too.[108] |
| 65 | Terbium | 1843 | G. Mosander | 1886 | J.C.G. de Marignac | Mosander managed to split the old yttria into yttria proper and erbia, and later terbia too.[109] |
| 44 | Ruthenium | 1844 | K. Claus | 1844 | K. Claus | Gottfried Wilhelm Osann thought that he found three new metals in Russian platinum samples, and in 1844 Karl Karlovich Klaus confirmed that there was a new element.[110] |
| 55 | Caesium | 1860 | R. Bunsen and R. Kirchhoff | 1882 | C. Setterberg | Bunsen and Kirchhoff were the first to suggest finding new elements by spectrum analysis. They discovered caesium by its two blue emission lines in a sample of Dürkheim mineral water.[111] The pure metal was eventually isolated in 1882 by Setterberg.[112] |
| 37 | Rubidium | 1861 | R. Bunsen and G. R. Kirchhoff | Hevesy | Bunsen and Kirchhoff discovered it just a few months after caesium, by observing new spectral lines in the mineral lepidolite. Bunsen never obtained a pure sample of the metal, which was later obtained by Hevesy.[113] | |
| 81 | Thallium | 1861 | W. Crookes | 1862 | C.-A. Lamy | Shortly after the discovery of rubidium, Crookes found a new green line in a selenium sample; later that year, Lamy found the element to be metallic.[114] |
| 49 | Indium | 1863 | F. Reich and T. Richter | 1867 | T. Richter | Reich and Richter first identified it in sphalerite by its bright indigo-blue spectroscopic emission line. Richter isolated the metal several years later.[115] |
| 2 | Helium | 1868 | N. Lockyer | 1895 | W. Ramsay, T. Cleve, and N. Langlet | P. Janssen and Lockyer observed independently a yellow line in the solar spectrum that did not match any other element. However, only Lockyer made the correct conclusion that it was due to a new element. This was the first observation of a noble gas, located in the Sun. Years later after the isolation of argon on Earth, Ramsay, Cleve, and Langlet observed independently helium trapped in cleveite.[116] |
| 1869 | D. I. Mendeleev | Mendeleev arranges the 64 elements known at that time into the first modern periodic table and correctly predicts several others. | ||||
| 31 | Gallium | 1875 | P. E. L. de Boisbaudran | P. E. L. de Boisbaudran | Boisbaudran observed on a pyrenea blende sample some emission lines corresponding to the eka-aluminium that was predicted by Mendeleev in 1871 and subsequently isolated the element by electrolysis.[117][118] | |
| 70 | Ytterbium | 1878 | J.C.G. de Marignac | 1906 | C. A. von Welsbach | On October 22, 1878, Marignac reported splitting terbia into two new earths, terbia proper and ytterbia.[119] |
| 67 | Holmium | 1878 | J.-L. Soret and M. Delafontaine | 1879 | T. Cleve | Soret found it in samarskite and later, Per Teodor Cleve split Marignac's erbia into erbia proper and two new elements, thulium and holmium. Delafontaine's philippium turned out to be identical to what Soret found.[120][121] |
| 69 | Thulium | 1879 | T. Cleve | 1879 | T. Cleve | Cleve split Marignac's erbia into erbia proper and two new elements, thulium and holmium.[122] |
| 21 | Scandium | 1879 | F. Nilson | 1879 | F. Nilson | Nilson split Marignac's ytterbia into pure ytterbia and a new element that matched Mendeleev's 1871 predicted eka-boron.[123] |
| 62 | Samarium | 1879 | P.E.L. de Boisbaudran | 1879 | P.E.L. de Boisbaudran | Boisbaudran noted a new earth in samarskite and named it samaria after the mineral.[124] |
| 64 | Gadolinium | 1880 | J. C. G. de Marignac | 1886 | P.E.L. de Boisbaudran | Marignac initially observed the new earth in terbia, and later Boisbaudran obtained a pure sample from samarskite.[125] |
| 59 | Praseodymium | 1885 | C. A. von Welsbach | Carl Auer von Welsbach discovered two new distinct elements in Mosander's didymia: praseodymium and neodymium.[126] | ||
| 60 | Neodymium | 1885 | C. A. von Welsbach | Carl Auer von Welsbach discovered two new distinct elements in Mosander's didymia: praseodymium and neodymium.[127] | ||
| 32 | Germanium | 1886 | C. A. Winkler | In February 1886 Winkler found in argyrodite the eka-silicon that Mendeleev had predicted in 1871.[128] | ||
| 66 | Dysprosium | 1886 | P.E.L. de Boisbaudran | 1905 | G. Urbain | De Boisbaudran found a new earth in erbia.[129] |
| 18 | Argon | 1894 | Lord Rayleigh and W. Ramsay | 1894 | Lord Rayleigh and W. Ramsay | They discovered the gas by comparing the molecular weights of nitrogen prepared by liquefaction from air and nitrogen prepared by chemical means. It is the first noble gas to be isolated.[130] |
| 63 | Europium | 1896 | E.-A. Demarçay | 1901 | E.-A. Demarçay | Demarçay found spectral lines of a new element in Lecoq's samarium, and separated this element several years later.[131] |
| 36 | Krypton | 1898 | W. Ramsay and W. Travers | 1898 | W. Ramsay and W. Travers | On May 30, 1898, Ramsay separated a noble gas from liquid argon by difference in boiling point.[132] |
| 10 | Neon | 1898 | W. Ramsay and W. Travers | 1898 | W. Ramsay and W. Travers | In June 1898 Ramsay separated a new noble gas from liquid argon by difference in boiling point.[132] |
| 54 | Xenon | 1898 | W. Ramsay and W. Travers | 1898 | W. Ramsay and W. Travers | On July 12, 1898 Ramsay separated a third noble gas within three weeks, from liquid argon by difference in boiling point.[133] |
| 84 | Polonium | 1898 | P. and M. Curie | 1902 | W. Marckwald | In an experiment done on July 13, 1898, the Curies noted an increased radioactivity in the uranium obtained from pitchblende, which they ascribed to an unknown element. Independently rediscovered and isolated in 1902 by Marckwald, who named it radiotellurium.[134] |
| 88 | Radium | 1898 | P. and M. Curie | 1902 | M. Curie | The Curies reported on December 26, 1898, a new element different from polonium, which Marie later isolated from uraninite.[135] |
| 86 | Radon | 1899 | E. Rutherford and R. B. Owens | 1910 | W. Ramsay and R. Whytlaw-Gray | Rutherford and Owens discovered a radioactive gas resulting from the radioactive decay of thorium, isolated later by Ramsay and Gray. In 1900, Friedrich Ernst Dorn discovered a longer-lived isotope of the same gas from the radioactive decay of radium. Since "radon" was first used to specifically designate Dorn's isotope before it became the name for the element, he is often mistakenly given credit for the latter instead of the former.[136][137] |
| 89 | Actinium | 1902 | F. O. Giesel | 1903 | F. O. Giesel | Giesel obtained from pitchblende a substance that had properties similar to those of lanthanum and named it emanium.[138] André-Louis Debierne had previously (in 1899 and 1900) reported the discovery of a new element actinium that was supposedly similar to titanium and thorium, which cannot have included much actual element 89. But by 1904, when Giesel and Debierne met, both had radiochemically pure element 89, and so Debierne has generally been given credit for the discovery.[139] |
| 71 | Lutetium | 1906 | C. A. von Welsbach and G. Urbain | 1906 | C. A. von Welsbach | von Welsbach proved that the old ytterbium also contained a new element, which he named cassiopeium. Urbain also proved this simultaneously, but his samples were very impure and only contained trace quantities of the new element. Despite this, his chosen name lutetium was adopted.[140] |
| 91 | Protactinium | 1913 | O. H. Göhring and K. Fajans | 1927 | A. von Grosse | The two obtained the first isotope of this element, 234mPa, that had been predicted by Mendeleev in 1871 as a member of the natural decay of 238U: they named it brevium. A longer-lived isotope 231Pa was found in 1918 by Otto Hahn and Lise Meitner, and was named by them protoactinium: since it is longer-lived, it gave the element its name. Protoactinium was changed to protactinium in 1949.[141] Originally isolated in 1900 by William Crookes, who nevertheless did not recognize that it was a new element.[142] |
| 72 | Hafnium | 1922 | D. Coster and G. von Hevesy | 1922 | D. Coster and G. von Hevesy | Georges Urbain claimed to have found the element in rare-earth residues, while Vladimir Vernadsky independently found it in orthite. Neither claim was confirmed due to World War I, and neither could be confirmed later, as the chemistry they reported does not match that now known for hafnium. After the war, Coster and Hevesy found it by X-ray spectroscopic analysis in Norwegian zircon.[143] |
| 75 | Rhenium | 1925 | W. Noddack, I. Noddack, O. Berg | 1928 | W. Noddack, I. Noddack | In 1925 Walter Noddack, Ida Eva Tacke and Otto Berg announced its separation from gadolinite and gave it the present name.[144][145] Masataka Ogawa had found it in thorianite in 1908, but assigned it as element 43 instead of 75 and named it nipponium.[146] Rhenium was the last stable element to be discovered. |
| 43 | Technetium | 1937 | C. Perrier and E. Segrè | 1937 | C. Perrier & E.Segrè | The two discovered a new element in a molybdenum sample that was used in a cyclotron, the first element to be discovered by synthesis. It had been predicted by Mendeleev in 1871 as eka-manganese.[147][148][149] In 1952, Paul W. Merrill found its spectral lines in S-type red giants.[150] Minuscule trace quantities were finally found on Earth in 1962 by B. T. Kenna and Paul K. Kuroda: they isolated it from Belgian Congo pitchblende, where it occurs as a spontaneous fission product of uranium.[151] |
| 87 | Francium | 1939 | M. Perey | Perey discovered it as a decay product of 227Ac.[152] Francium was the last element to be discovered in nature, rather than synthesized in the lab, although four of the "synthetic" elements that were discovered later (plutonium, neptunium, astatine, and promethium) were eventually found in trace amounts in nature as well.[153] | ||
| 93 | Neptunium | 1940 | E.M. McMillan and H. Abelson | Obtained by irradiating uranium with neutrons, it was the first transuranium element discovered.[154] Natural traces were found in Belgian Congo pitchblende by D. F. Peppard et al. in 1952.[155] | ||
| 85 | Astatine | 1940 | R. Corson, R. MacKenzie and E. Segrè | Obtained by bombarding bismuth with alpha particles.[156] In 1943, Berta Karlik and Traude Bernert found it in nature; due to World War II, they were initially unaware of Corson et al.'s results.[157] | ||
| 94 | Plutonium | 1940–1941 | Glenn T. Seaborg, Arthur C. Wahl, W. Kennedy and E.M. McMillan | Prepared by bombardment of uranium with deuterons.[158] Seaborg and Morris L. Perlman then found it as traces in natural Canadian pitchblende in 1941–1942, though this work was kept secret until 1948.[159] | ||
| 96 | Curium | 1944 | Glenn T. Seaborg, Ralph A. James and Albert Ghiorso | Prepared by bombarding plutonium with alpha particles during the Manhattan Project[160] | ||
| 95 | Americium | 1944 | G. T. Seaborg, R. A. James, O. Morgan and A. Ghiorso | Prepared by irradiating plutonium with neutrons during the Manhattan Project.[161] | ||
| 61 | Promethium | 1945 | Charles D. Coryell, Jacob A. Marinsky, and Lawrence E. Glendenin | 1945 | Charles D. Coryell, Jacob A. Marinsky, and Lawrence E. Glendenin[162][163] | It was probably first prepared at the Ohio State University in 1942 by bombarding neodymium and praseodymium with neutrons, but separation of the element could not be carried out. Isolation was performed under the Manhattan Project in 1945.[164] Found on Earth in trace quantities by Olavi Erämetsä in 1965; so far, promethium is the most recent element to have been found on Earth.[165] |
| 97 | Berkelium | 1949 | G. Thompson, A. Ghiorso and G. T. Seaborg (University of California, Berkeley) | Created by bombardment of americium with alpha particles.[166] | ||
| 98 | Californium | 1950 | S. G. Thompson, K. Street, Jr., A. Ghiorso and G. T. Seaborg (University of California, Berkeley) | Bombardment of curium with alpha particles.[167] | ||
| 99 | Einsteinium | 1952 | A. Ghiorso et al. (Argonne Laboratory, Los Alamos Laboratory and University of California, Berkeley) | 1952 | Formed in the first thermonuclear explosion in November 1952, by irradiation of uranium with neutrons; kept secret for several years.[168] | |
| 100 | Fermium | 1952 | A. Ghiorso et al. (Argonne Laboratory, Los Alamos Laboratory and University of California, Berkeley) | Formed in the first thermonuclear explosion in November 1952, by irradiation of uranium with neutrons; kept secret for several years.[169] | ||
| 101 | Mendelevium | 1955 | A. Ghiorso, G. Harvey, G. R. Choppin, S. G. Thompson and G. T. Seaborg (Berkeley Radiation Laboratory) | Prepared by bombardment of einsteinium with helium.[170] | ||
| 103 | Lawrencium | 1961 | A. Ghiorso, T. Sikkeland, E. Larsh and M. Latimer (Berkeley Radiation Laboratory) | First prepared by bombardment of californium with boron atoms.[171] | ||
| 102 | Nobelium | 1966 | E. D. Donets, V. A. Shchegolev and V. A. Ermakov (JINR in Dubna) | First prepared by bombardment of uranium with neon atoms[172] | ||
| 104 | Rutherfordium | 1969 | A. Ghiorso et al. (Berkeley Radiation Laboratory) and I. Zvara et al. (JINR in Dubna) | Prepared by bombardment of californium with carbon atoms by Albert Ghiorso's team and by bombardment of plutonium with neon atoms by Zvara's team.[173] | ||
| 105 | Dubnium | 1970 | A. Ghiorso et al. (Berkeley Radiation Laboratory) and V. A. Druin et al. (JINR in Dubna) | Prepared by bombardment of californium with nitrogen atoms by Ghiorso's team and by bombardment of americium with neon atoms by Druin's team.[174] | ||
| 106 | Seaborgium | 1974 | A. Ghiorso et al. (Berkeley Radiation Laboratory) | Prepared by bombardment of californium with oxygen atoms.[175] | ||
| 107 | Bohrium | 1981 | G.Münzenberg et al. (GSI in Darmstadt) | Obtained by bombarding bismuth with chromium.[176] | ||
| 109 | Meitnerium | 1982 | G. Münzenberg, P. Armbruster et al. (GSI in Darmstadt) | Prepared by bombardment of bismuth with iron atoms.[177] | ||
| 108 | Hassium | 1984 | G. Münzenberg, P. Armbruster et al. (GSI in Darmstadt) | Prepared by bombardment of lead with iron atoms[178] | ||
| 110 | Darmstadtium | 1994 | S. Hofmann et al. (GSI in Darmstadt) | Prepared by bombardment of lead with nickel[179] | ||
| 111 | Roentgenium | 1994 | S. Hofmann et al. (GSI in Darmstadt) | Prepared by bombardment of bismuth with nickel[180] | ||
| 112 | Copernicium | 1996 | S. Hofmann et al. (GSI in Darmstadt) | Prepared by bombardment of lead with zinc.[181][182] | ||
| 114 | Flerovium | 1998 | Y. Oganessian et al. (JINR in Dubna) | Prepared by bombardment of plutonium with calcium[183] | ||
| 116 | Livermorium | 2000 | Y. Oganessian et al. (JINR in Dubna) | Prepared by bombardment of curium with calcium[184] | ||
| 118 | Oganesson | 2002 | Y. Oganessian et al. (JINR in Dubna) | Prepared by bombardment of californium with calcium[185] | ||
| 115 | Moscovium | 2003 | Y. Oganessian et al. (JINR in Dubna) | Prepared by bombardment of americium with calcium[186] | ||
| 113 | Nihonium | 2003–2004 | Y. Oganessian et al. (JINR in Dubna) and K. Morita et al. (RIKEN in Wako, Japan) | Prepared by decay of moscovium by Oganessian's team[186] and bombardment of bismuth with zinc by Morita's team.[187] Both teams began their experiments in 2003; Oganessian's team detected its first atom in 2003, but Morita's only in 2004. However, both teams published in 2004. | ||
| 117 | Tennessine | 2009 | Y. Oganessian et al. (JINR in Dubna) | Prepared by bombardment of berkelium with calcium[188] | ||
Graphics
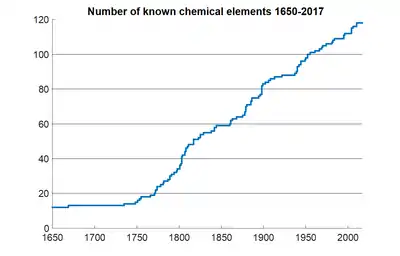
Graph of number of known chemical elements from 1650 until present
See also
- History of the periodic table
- Periodic table
- Extended periodic table
- The Mystery of Matter: Search for the Elements (2014/2015 PBS film)
- Transfermium Wars
References
- "Copper History". Rameria.com. Archived from the original on 2008-09-17. Retrieved 2008-09-12.
- "CSA – Discovery Guides, A Brief History of Copper".
- "Serbian site may have hosted first copper makers". UCL.ac.uk. UCL Institute of Archaeology. 23 September 2010. Retrieved 22 April 2017.
- Bruce Bower (July 17, 2010). "Serbian site may have hosted first copper makers". ScienceNews. Retrieved 22 April 2017.
- "The History of Lead – Part 3". Lead.org.au. Archived from the original on 2004-10-18. Retrieved 2008-09-12.
- Gopher, A.; Tsuk, T.; Shalev, S. & Gophna, R. (August–October 1990). "Earliest Gold Artifacts in the Levant". Current Anthropology. 31 (4): 436–443. doi:10.1086/203868. JSTOR 2743275. S2CID 143173212.
- "47 Silver".
- "Silver Facts – Periodic Table of the Elements". Chemistry.about.com. Retrieved 2008-09-12.
- "26 Iron". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- Weeks, Mary Elvira; Leichester, Henry M. (1968). "Elements Known to the Ancients". Discovery of the Elements. Easton, PA: Journal of Chemical Education. pp. 29–40. ISBN 0-7661-3872-0. LCCN 68-15217.
- "Notes on the Significance of the First Persian Empire in World History". Courses.wcupa.edu. Retrieved 2008-09-12.
- "History of Carbon and Carbon Materials – Center for Applied Energy Research – University of Kentucky". Caer.uky.edu. Archived from the original on 2012-11-01. Retrieved 2008-09-12.
- "Chinese made first use of diamond". BBC News. 17 May 2005. Retrieved 2007-03-21.
- Ferchault de Réaumur, R-A (1722). L'art de convertir le fer forgé en acier, et l'art d'adoucir le fer fondu, ou de faire des ouvrages de fer fondu aussi finis que le fer forgé (English translation from 1956). Paris, Chicago.
- Senese, Fred (September 9, 2009). "Who discovered carbon?". Frostburg State University. Retrieved 2007-11-24.
- "50 Tin". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- Hauptmann, A.; Maddin, R.; Prange, M. (2002), "On the structure and composition of copper and tin ingots excavated from the shipwreck of Uluburun", Bulletin of the American School of Oriental Research, American Schools of Oriental Research, vol. 328, no. 328, pp. 1–30, JSTOR 1357777
- "History of Metals". Neon.mems.cmu.edu. Archived from the original on 2007-01-08. Retrieved 2008-09-12.
- "Sulfur History". Georgiagulfsulfur.com. Archived from the original on 2008-09-16. Retrieved 2008-09-12.
- Rapp, George Robert (4 February 2009). Archaeomineralogy. p. 242. ISBN 978-3-540-78593-4.
- Kraus, Paul (1942–1943). Jâbir ibn Hayyân: Contribution à l'histoire des idées scientifiques dans l'Islam. I. Le corpus des écrits jâbiriens. II. Jâbir et la science grecque. Cairo: Institut Français d'Archéologie Orientale. ISBN 9783487091150. OCLC 468740510. vol. II, p. 1, note 1; Weisser, Ursula (1980). Spies, Otto (ed.). Das "Buch über das Geheimnis der Schöpfung" von Pseudo-Apollonios von Tyana. Berlin: De Gruyter. doi:10.1515/9783110866933. ISBN 978-3-11-007333-1. p. 199. On the dating and historical background of the Sirr al-khalīqa, see Kraus 1942−1943, vol. II, pp. 270–303; Weisser 1980, pp. 39–72. On the dating of the writings attributed to Jābir, see Kraus 1942−1943, vol. I, pp. xvii–lxv. A more detailed and speculative account of the sulfur-mercury theory of metals is given by Holmyard, E.J. (1931). Makers of Chemistry. Oxford: Clarendon Press. pp. 57–58.
- "Mercury and the environment – Basic facts". Environment Canada, Federal Government of Canada. 2004. Archived from the original on 2007-01-15. Retrieved 2008-03-27.
- Craddock, P. T. et al. (1983), "Zinc production in medieval India", World Archaeology 15 (2), Industrial Archaeology, p. 13
- "30 Zinc". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- Weeks, Mary Elvira (1933). "III. Some Eighteenth-Century Metals". The Discovery of the Elements. Easton, PA: Journal of Chemical Education. p. 21. ISBN 0-7661-3872-0.
- David A. Scott and Warwick Bray (1980). "Ancient Platinum Technology in South America: Its use by the Indians in Pre-Hispanic Times". Platinum Metals Review. Retrieved 5 Nov 2018.
- "78 Platinum". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- Holmyard, Eric John (1957). Alchemy. Courier Corporation. ISBN 9780486262987. Retrieved 26 January 2018.
- Sarton, George (1927–1948). Introduction to the History of Science. Vol. I–III. Baltimore: Williams & Wilkins. OCLC 476555889. vol. I, p. 532: "We find in them [sc. the works attributed to Jabir] [...] preparation of various substances (e. g., basic lead carbonate; arsenic and antimony from their sulphides)." On the dating of the writings attributed to Jabir, see Kraus 1942–1943, vol. I, pp. xvii–lxv.
- Emsley, John (2001). Nature's Building Blocks: An A-Z Guide to the Elements. Oxford University Press. ISBN 9780198503415. Retrieved 28 February 2018.
- Healy, John F. (1999). Pliny the Elder on Science and Technology. Oxford University Press. ISBN 9780198146872. Retrieved 26 January 2018.
- Biringuccio, Vannoccio (1959). Pirotechnia. Courier Corporation. pp. 91–92. ISBN 9780486261348. Retrieved 31 January 2018.
Probably metallic antimony was being produced in Germany in Biringuccio's time, for later in this chapter he mentions importation of cakes of the smelted (or melted) metal to alloy with pewter or bell metal.
- Bismuth - Royal Society of Chemistry
- Gordon, Robert B.; Rutledge, John W. (1984). "Bismuth Bronze from Machu Picchu, Peru". Science. 223 (4636): 585–586. Bibcode:1984Sci...223..585G. doi:10.1126/science.223.4636.585. JSTOR 1692247. PMID 17749940. S2CID 206572055.
- Agricola, Georgious (1955) [1546]. De Natura Fossilium. New York: Mineralogical Society of America. p. 178.
- Nicholson, William (1819). "Bismuth". American edition of the British encyclopedia: Or, Dictionary of Arts and sciences; comprising an accurate and popular view of the present improved state of human knowledge. p. 181.
- Weeks, Mary Elvira (1932). "The discovery of the elements. II. Elements known to the alchemists". Journal of Chemical Education. 9 (1): 11. Bibcode:1932JChEd...9...11W. doi:10.1021/ed009p11.
- Giunta, Carmen J. "Glossary of Archaic Chemical Terms". Le Moyne College. See also for other terms for bismuth, including stannum glaciale (glacial tin or ice-tin).
- Pott, Johann Heinrich (1738). "De Wismutho". Exercitationes Chymicae. Berolini: Apud Johannem Andream Rüdigerum. p. 134.
- Geoffroy, C.F. (1753). "Sur Bismuth". Histoire de l'Académie Royale des Sciences ... Avec les Mémoires de Mathématique & de Physique ... Tirez des Registres de Cette Académie: 190.
- "15 Phosphorus". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- "27 Cobalt". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- "28 Nickel". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- "01 Hydrogen". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- Andrews, A. C. (1968). "Oxygen". In Clifford A. Hampel (ed.). The Encyclopedia of the Chemical Elements. New York: Reinhold Book Corporation. pp. 272. LCCN 68-29938.
- "08 Oxygen". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- Cook, Gerhard A.; Lauer, Carol M. (1968). "Oxygen". In Clifford A. Hampel (ed.). The Encyclopedia of the Chemical Elements. New York: Reinhold Book Corporation. pp. 499–500. LCCN 68-29938.
- Stasińska, Grażyna (2012). "The discovery of oxygen in the universe" (PDF). ppgfsc.posgrad.ufsc.br. Retrieved 20 April 2018.
- Roza, Greg (2010). The Nitrogen Elements: Nitrogen, Phosphorus, Arsenic, Antimony, Bismuth. p. 7. ISBN 9781435853355.
- "07 Nitrogen". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- "17 Chlorine". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- "25 Manganese". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- "42 Molybdenum". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- IUPAC. "74 Tungsten". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- "52 Tellurium". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- "Lavoisier 1789 – 33 elements". Elementymology & Elements Multidict. Retrieved 2015-01-24.
- M. H. Klaproth (1789). "Chemische Untersuchung des Uranits, einer neuentdeckten metallischen Substanz". Chemische Annalen. 2: 387–403.
- E.-M. Péligot (1842). "Recherches Sur L'Uranium". Annales de chimie et de physique. 5 (5): 5–47.
- "Titanium". Los Alamos National Laboratory. 2004. Archived from the original on 2006-12-30. Retrieved 2006-12-29.
- Barksdale, Jelks (1968). The Encyclopedia of the Chemical Elements. Skokie, Illinois: Reinhold Book Corporation. pp. 732–38 "Titanium". LCCCN 68-29938.
- Vauquelin, Louis Nicolas (1798). "Memoir on a New Metallic Acid which exists in the Red Lead of Sibiria". Journal of Natural Philosophy, Chemistry, and the Arts. 3: 146.
- Glenn, William (1896). "Chrome in the Southern Appalachian Region". Transactions of the American Institute of Mining, Metallurgical and Petroleum Engineers. 25: 482.
- "41 Niobium". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- "73 Tantalum". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- "46 Palladium". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- "58 Cerium". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- "76 Osmium". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- "77 Iridium". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- "45 Rhodium". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- "19 Potassium". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- "11 Sodium". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- "05 Boron". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- "56 Barium". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- "38 Strontium". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- "12 Magnesium". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- Bache, Franklin (1819). A System of Chemistry for the Use of Students of Medicine. Philadelphia: William Fry. p. 135. ISBN 9780608435060.
- Davy, Humphry (1812). Elements of Chemical Philosophy. London: W. Bulmer and Co. Cleveland-row. pp. 362–364. ISBN 9780598818836.
- "14 Silicon". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- "Silicon". The Environmental Literacy Council. Retrieved 2016-12-02.
- Bache, Franklin (1819). A System of Chemistry for the Use of Students of Medicine. Philadelphia: William Fry. p. 135. ISBN 9780608435060.
- Davy, Humphry (1812). Elements of Chemical Philosophy. London: W. Bulmer and Co. Cleveland-row. pp. 354–357. ISBN 9780598818836.
- "13 Aluminium". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- Örsted, H. C. (1825). Oversigt over det Kongelige Danske Videnskabernes Selskabs Forhanlingar og dets Medlemmerz Arbeider, fra 31 Mai 1824 til 31 Mai 1825 [Overview of the Royal Danish Science Society's Proceedings and the Work of its Members, from 31 May 1824 to 31 May 1825] (in Danish). pp. 15–16.
- "40 Zirconium". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- Lide, David R., ed. (2007–2008). "Zirconium". CRC Handbook of Chemistry and Physics. Vol. 4. New York: CRC Press. p. 42. ISBN 978-0-8493-0488-0.
- Bache, Franklin (1819). A System of Chemistry for the Use of Students of Medicine. Philadelphia: William Fry. p. 135. ISBN 9780608435060.
- Davy, Humphry (1812). Elements of Chemical Philosophy. London: W. Bulmer and Co. Cleveland-row. pp. 360–362. ISBN 9780598818836.
- Bache, Franklin (1819). A System of Chemistry for the Use of Students of Medicine. Philadelphia: William Fry. p. 135. ISBN 9780608435060.
- Davy, Humphry (1812). Elements of Chemical Philosophy. London: W. Bulmer and Co. Cleveland-row. pp. 358–359. ISBN 9780598818836.
- "04 Beryllium". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- Browning, Philip Embury (1917). "Introduction to the Rarer Elements". Kongl. Vet. Acad. Handl. XV: 137.
- Gadolin, Johan (1796). "Von einer schwarzen, schweren Steinart aus Ytterby Steinbruch in Roslagen in Schweden". Crell's Annalen. I: 313–329.
- Bache, Franklin (1819). A System of Chemistry for the Use of Students of Medicine. Philadelphia: William Fry. p. 135. ISBN 9780608435060.
- Davy, Humphry (1812). Elements of Chemical Philosophy. London: W. Bulmer and Co. Cleveland-row. pp. 364–366. ISBN 9780598818836.
- Heiserman, David L. (1992). "Element 39: Yttrium". Exploring Chemical Elements and their Compounds. New York: TAB Books. pp. 150–152. ISBN 0-8306-3018-X.
- Wöhler, Friedrich (1828). "Ueber das Beryllium und Yttrium". Annalen der Physik. 89 (8): 577–582. Bibcode:1828AnP....89..577W. doi:10.1002/andp.18280890805.
- "09 Fluorine". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- "53 Iodine". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- "03 Lithium". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- "48 Cadmium". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- "34 Selenium". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- "35 Bromine". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- Carl Löwig (1827) "Über Brombereitung und eine auffallende Zersetzung des Aethers durch Chlor" (On the preparation of bromine and a striking decomposition of ether by chlorine), Magazine für Pharmacie, vol. 21, pages 31–36.
- "90 Thorium". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- "23 Vanadium". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- "57 Lanthanum". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- "Erbium". RSC.org. Retrieved 2016-12-02.
- "Terbium". RSC.org. Retrieved 2016-12-02.
- "44 Ruthenium". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- "55 Caesium". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- Caesium Archived 2012-03-09 at the Wayback Machine
- "37 Rubidium". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- "81 Thallium". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- "49 Indium". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- "02 Helium". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- "31 Gallium". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- "The New Metal Gallium". Scientific American. June 15, 1878. Retrieved 2016-06-16.
- "70 Ytterbium". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- "67 Holmium". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- Fontani, Marco; Costa, Mariagrazia; Orna, Mary Virginia (2014). The Lost Elements: The Periodic Table's Shadow Side. Oxford University Press. p. 123. ISBN 9780199383344.
...today's inclination to re-evaluate the work of Delafontaine and Soret has led justifiably to their being included as co-discoverers of holmium.
- "69 Thulium". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- "21 Scandium". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- "62 Samarium". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- "64 Gadolinium". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- "59 Praseodymium". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- "60 Neodymium". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- "32 Germanium". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- "66 Dysprosium". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- "18 Argon". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- "63 Europium". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- "10 Neon". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- "54 Xenon". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- "84 Polonium". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- "88 Radium". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- Partington, J. R. (May 1957). "Discovery of Radon". Nature. 179 (4566): 912. Bibcode:1957Natur.179..912P. doi:10.1038/179912a0. S2CID 4251991.
- Ramsay, W.; Gray, R. W. (1910). "La densité de l'emanation du radium". Comptes rendus hebdomadaires des séances de l'Académie des sciences. 151: 126–128.
- "89 Actinium". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- Kirby, Harold W. (1971). "The Discovery of Actinium". Isis. 62 (3): 290–308. doi:10.1086/350760. JSTOR 229943. S2CID 144651011.
- "71 Lutetium". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- "91 Protactinium". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- Emsley, John (2001). Nature's Building Blocks ((Hardcover, First Edition) ed.). Oxford University Press. pp. 347. ISBN 0-19-850340-7.
- "72 Hafnium". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- Noddack, W.; Tacke, I.; Berg, O (1925). "Die Ekamangane". Naturwissenschaften. 13 (26): 567. Bibcode:1925NW.....13..567.. doi:10.1007/BF01558746. S2CID 32974087.
- "75 Rhenium". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- "Archived copy" (PDF). Archived from the original (PDF) on 2008-10-03. Retrieved 2008-07-11.
{{cite web}}: CS1 maint: archived copy as title (link) - "43 Technetium". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- History of the Origin of the Chemical Elements and Their Discoverers, Individual Element Names and History, "Technetium"
- "Chemical Elements Discovered at Lawrence Berkeley National Lab". Lawrence Berkeley National Laboratory. Retrieved 2017-03-02.
- Merrill, P. W. (1952). "Technetium in the stars". Science. 115 (2992): 479–489 [484]. Bibcode:1952Sci...115..479.. doi:10.1126/science.115.2992.479. PMID 17792758.
- Kenna, B. T.; Kuroda, P. K. (1964). "Technetium in Nature". Journal of Inorganic and Nuclear Chemistry. 26 (4): 493–499. doi:10.1016/0022-1902(64)80280-3.
- "87 Francium". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- Adloff, Jean-Pierre; Kaufman, George B. (2005-09-25). Francium (Atomic Number 87), the Last Discovered Natural Element Archived June 4, 2013, at the Wayback Machine. The Chemical Educator 10 (5). [2007-03-26]
- "93 Neptunium". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- Peppard, D. F.; Mason, G. W.; Gray, P. R.; Mech, J. F. (1952). "Occurrence of the (4n + 1) series in nature" (PDF). Journal of the American Chemical Society. 74 (23): 6081–6084. doi:10.1021/ja01143a074.
- "85 Astatine". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- Burdette, S. C.; Thornton, B. F. (2010). "Finding Eka-Iodine: Discovery Priority in Modern Times" (PDF). Bulletin for the History of Chemistry. 35: 86–96.
- "94 Plutonium". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- Seaborg, Glenn T.; Perlman, Morris L. (1948). "Search for Elements 94 and 93 in Nature. Presence of 94239 in Pitchblende". J. Am. Chem. Soc. 70 (4): 1571–1573. doi:10.1021/ja01184a083. PMID 18915775.
- "96 Curium". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- "95 Americium". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- Marinsky, J. A.; Glendenin, L. E.; Coryell, C. D. (1947). "The chemical identification of radioisotopes of neodymium and of element 61". Journal of the American Chemical Society. 69 (11): 2781–5. doi:10.1021/ja01203a059. hdl:2027/mdp.39015086506477. PMID 20270831.
- "Discovery of Promethium" (PDF). Oak Ridge National Laboratory Review. 36 (1): 3. 2003. Retrieved 2018-06-17.
- "61 Promethium". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- McGill, Ian. "Rare Earth Elements". Ullmann's Encyclopedia of Industrial Chemistry. Vol. 31. Weinheim: Wiley-VCH. p. 188. doi:10.1002/14356007.a22_607.
- "97 Berkelium". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- "98 Californium". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- "99 Einsteinium". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- "100 Fermium". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- "101 Mendelevium". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- "103 Lawrencium". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- "102 Nobelium". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- "104 Rutherfordium". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- "105 Dubnium". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- "106 Seaborgium". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- "107 Bohrium". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- "109 Meitnerium". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- "108 Hassium". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- "110 Darmstadtium". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- "111 Roentgenium". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- "112 Copernicium". Elements.vanderkrogt.net. Retrieved 2009-07-17.
- "Discovery of the Element with Atomic Number 112". www.iupac.org. 2009-06-26. Archived from the original on 2009-12-21. Retrieved 2009-07-17.
- Oganessian, Yu. Ts.; Utyonkov, V. K.; Lobanov, Yu. V.; Abdullin, F. Sh.; Polyakov, A. N.; Shirokovsky, I. V.; Tsyganov, Yu. S.; Gulbekian, G. G.; Bogomolov, S. L.; Gikal, B.; Mezentsev, A.; Iliev, S.; Subbotin, V.; Sukhov, A.; Buklanov, G.; Subotic, K.; Itkis, M.; Moody, K.; Wild, J.; Stoyer, N.; Stoyer, M.; Lougheed, R. (October 1999). "Synthesis of Superheavy Nuclei in the 48Ca + 244Pu Reaction". Physical Review Letters. 83 (16): 3154. Bibcode:1999PhRvL..83.3154O. doi:10.1103/PhysRevLett.83.3154. S2CID 109929705.
- Oganessian, Yu. Ts.; Utyonkov, V. K.; Lobanov, Yu. V.; Abdullin, F. Sh.; Polyakov, A. N.; Shirokovsky, I. V.; Tsyganov, Yu. S.; Gulbekian, G. G.; Bogomolov, S. L.; Gikal, B.; Mezentsev, A.; Iliev, S.; Subbotin, V.; Sukhov, A.; Ivanov, O.; Buklanov, G.; Subotic, K.; Itkis, M.; Moody, K.; Wild, J.; Stoyer, N.; Stoyer, M.; Lougheed, R.; Laue, C.; Karelin, Ye.; Tatarinov, A. (2000). "Observation of the decay of 292116". Physical Review C. 63 (1): 011301. Bibcode:2000PhRvC..63a1301O. doi:10.1103/PhysRevC.63.011301.
- Oganessian, Yu. Ts.; Utyonkov, V. K.; Lobanov, Yu. V.; Abdullin, F. Sh.; Polyakov, A. N.; Sagaidak, R. N.; Shirokovsky, I. V.; Tsyganov, Yu. S.; Voinov, A. A.; Gulbekian, G.; Bogomolov, S.; Gikal, B.; Mezentsev, A.; Iliev, S.; Subbotin, V.; Sukhov, A.; Subotic, K.; Zagrebaev, V.; Vostokin, G.; Itkis, M.; Moody, K.; Patin, J.; Shaughnessy, D.; Stoyer, M.; Stoyer, N.; Wilk, P.; Kenneally, J.; Landrum, J.; Wild, J.; Lougheed, R. (2006). "Synthesis of the isotopes of elements 118 and 116 in the 249Cf and 245Cm+48Ca fusion reactions". Physical Review C. 74 (4): 044602. Bibcode:2006PhRvC..74d4602O. doi:10.1103/PhysRevC.74.044602.
- Oganessian, Yu. Ts.; Utyonkov, V. K.; Dmitriev, S. N.; Lobanov, Yu. V.; Itkis, M. G.; Polyakov, A. N.; Tsyganov, Yu. S.; Mezentsev, A. N.; Yeremin, A. V.; Voinov, A.; Sokol, E.; Gulbekian, G.; Bogomolov, S.; Iliev, S.; Subbotin, V.; Sukhov, A.; Buklanov, G.; Shishkin, S.; Chepygin, V.; Vostokin, G.; Aksenov, N.; Hussonnois, M.; Subotic, K.; Zagrebaev, V.; Moody, K.; Patin, J.; Wild, J.; Stoyer, M.; Stoyer, N.; et al. (2005). "Synthesis of elements 115 and 113 in the reaction 243Am + 48Ca". Physical Review C. 72 (3): 034611. Bibcode:2005PhRvC..72c4611O. doi:10.1103/PhysRevC.72.034611.
- Morita, Kosuke; Morimoto, Kouji; Kaji, Daiya; Akiyama, Takahiro; Goto, Sin-ichi; Haba, Hiromitsu; Ideguchi, Eiji; Kanungo, Rituparna; Katori, Kenji; Koura, Hiroyuki; Kudo, Hisaaki; Ohnishi, Tetsuya; Ozawa, Akira; Suda, Toshimi; Sueki, Keisuke; Xu, HuShan; Yamaguchi, Takayuki; Yoneda, Akira; Yoshida, Atsushi; Zhao, YuLiang (2004). "Experiment on the Synthesis of Element 113 in the Reaction 209Bi(70Zn,n)278113". Journal of the Physical Society of Japan. 73 (10): 2593–2596. Bibcode:2004JPSJ...73.2593M. doi:10.1143/JPSJ.73.2593.
- Oganessian, Yu. Ts.; Abdullin, F. Sh.; Bailey, P. D.; Benker, D. E.; Bennett, M. E.; Dmitriev, S. N.; Ezold, J. G.; Hamilton, J. H.; Henderson, R. A.; Itkis, M. G.; Lobanov, Yu. V.; Mezentsev, A. N.; Moody, K. J.; Nelson, S. L.; Polyakov, A. N.; Porter, C. E.; Ramayya, A. V.; Riley, F. D.; Roberto, J. B.; Ryabinin, M. A.; Rykaczewski, K. P.; Sagaidak, R. N.; Shaughnessy, D. A.; Shirokovsky, I. V.; Stoyer, M. A.; Subbotin, V. G.; Sudowe, R.; Sukhov, A. M.; Tsyganov, Yu. S.; et al. (April 2010). "Synthesis of a New Element with Atomic Number Z=117". Physical Review Letters. 104 (14): 142502. Bibcode:2010PhRvL.104n2502O. doi:10.1103/PhysRevLett.104.142502. PMID 20481935.
External links
- History of the Origin of the Chemical Elements and Their Discoverers Last updated by Boris Pritychenko on March 30, 2004
- History of Elements of the Periodic Table
- Timeline of Element Discoveries
- The Historyscoper
- Discovery of the Elements – The Movie – YouTube (1:18)
- The History Of Metals Timeline. A timeline showing the discovery of metals and the development of metallurgy.
- —Eric Scerri, 2007, The periodic table: Its story and its significance, Oxford University Press, New York, ISBN 9780195305739
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.