Enantiomer
In chemistry, an enantiomer (/ɪˈnænti.əmər, ɛ-, -oʊ-/[1] ih-NAN-tee-ə-mər; from Ancient Greek ἐνάντιος (enántios) 'opposite', and μέρος (méros) 'part') – also called optical isomer,[2] antipode,[3] or optical antipode[4] – is one of two stereoisomers that are mirror images of each other that are non-superposable (not identical), much as one's left and right hands are mirror images of each other that cannot appear identical simply by reorientation.[5] A single chiral atom or similar structural feature in a compound causes that compound to have two possible structures which are non-superposable, each a mirror image of the other. Each member of the pair is termed an enantiomorph (enantio = opposite; morph = form);[6] the structural property is termed enantiomerism. The presence of multiple chiral features in a given compound increases the number of geometric forms possible, though there may still be some perfect-mirror-image pairs.
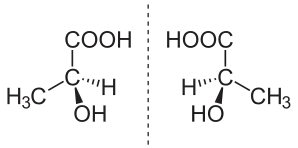
A sample of a chemical is considered enantiopure (also termed enantiomerically pure) when it has, within the limits of detection, molecules of only one chirality.[7]
When present in a symmetric environment, enantiomers have identical chemical and physical properties except for their ability to rotate plane-polarized light (+/−) by equal amounts but in opposite directions (although the polarized light can be considered an asymmetric medium). Such compounds are therefore described as optically active, with specific terms for each enantiomer based on the direction: a dextrorotatory compound rotates light a clockwise (+) direction whereas a levorotatory compound rotates light in a counter-clockwise (–) direction. A mixture of equal number of both enantiomers is called a racemic mixture or a racemate.[8] In a racemic mixture, the amount of positive rotation is exactly counteracted by the equal amount of negative rotation, so the net rotation is zero (the mixture is not optically active).
Enantiomer members often have different chemical reactions with other enantiomer substances. Since many biological molecules are enantiomers, there is sometimes a marked difference in the effects of two enantiomers on biological organisms. In drugs, for example, often only one of a drug's enantiomers is responsible for the desired physiological effects (referred to as eutomer),[9] while the other enantiomer is less active, inactive, or sometimes even productive of adverse effects (referred to as distomer).[10] Owing to this discovery, drugs composed of only one enantiomer ("enantiopure") can be developed to make the drug work better and sometimes eliminate some side effects. An example is eszopiclone (Lunesta), which is just a single enantiomer of an older racemic drug called zopiclone. This is a typical example of a chiral switch. One enantiomer is responsible for all the desired effects, while the other enantiomer seems to be inactive, so the dose of eszopiclone is half that of zopiclone.
In chemical synthesis of enantiomeric substances, non-enantiomeric precursors inevitably produce racemic mixtures. In the absence of an effective enantiomeric environment (precursor, chiral catalyst, or kinetic resolution), separation of a racemic mixture into its enantiomeric components is impossible, although certain racemic mixtures spontaneously crystallize in the form of a racemic conglomerate, in which crystals of the enantiomers are physically segregated and may be separated mechanically (e.g., the enantiomers of tartaric acid, whose crystallized enantiomers were separated with tweezers by Pasteur). However, most racemates will crystallize in crystals containing both enantiomers in a 1:1 ratio, arranged in a regular lattice.
Naming conventions
The R/S system is an important nomenclature system used to denote distinct enantiomers. Another naming system uses the prefixes (+)- and (−)- to denote the enantiomer's optical activity. (Prefixes d- and l- are obsolete synonyms for (+)- and (−)-). The Latin words for left are laevus and sinister, and the word for right is dexter (or rectus in the sense of correct or virtuous). The English word right is a cognate of rectus. This is the origin of the L/D and S/R notations, and the employment of prefixes levo- and dextro- in common names.
The prefix ent- to a chemical name can be used to refer to the chemical that is the enantiomer of the one indicated by the name.[11]
Chirality centers
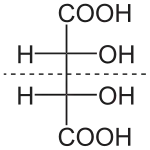
A molecule is chiral, and therefore possesses an enantiomer, if it has no reflection symmetry and no improper rotation (rotoreflection) symmetry. A common characteristic of chirality is the presence of an asymmetric carbon atom, which has bonds to four different atoms or groups, so that these groups can be arranged in two different ways that are not superposable. Having an enantiomer by virtue of an asymmetric carbon atom (or asymmetrically-bonded atom of any element) represents a common type of chirality, called point chirality or central chirality.[12]: pg. 2 The asymmetric atom is called a chirality center,[13][14] a type of stereocenter. A chirality center is also called a chiral center[12][15][16] or an asymmetric center.[17] Some sources use the terms stereocenter, stereogenic center, stereogenic atom or stereogen to refer exclusively to a chirality center,[12][16][18] while others use the terms more broadly to refer also to centers that result in diastereomers (stereoisomers that are not enantiomers).[14][19][20]
Compounds that contain exactly one (or any odd number) of asymmetric atoms are always chiral. However, compounds that contain an even number of asymmetric atoms sometimes lack chirality because they are arranged in mirror-symmetric pairs, and are known as meso compounds. For instance, meso tartaric acid (shown on the right) has two asymmetric carbon atoms, but it does not exhibit enantiomerism because there is a mirror symmetry plane. Conversely, there exist forms of chirality that do not require asymmetric atoms, such as axial, planar, and helical chirality.[12]: pg. 3
Even though a chiral molecule lacks reflection (Cs) and rotoreflection symmetries (S2n), it can have other molecular symmetries, and its symmetry is described by one of the chiral point groups: Cn, Dn, T, O, or I. For example, hydrogen peroxide is chiral and has C2 (two-fold rotational) symmetry. A common chiral case is the point group C1, meaning no symmetries, which is the case for lactic acid.
Examples
-Mecoprop_Enantiomers_Formulae.png.webp)
An example of such an enantiomer is the sedative thalidomide, which was sold in a number of countries around the world from 1957 until 1961. It was withdrawn from the market when it was found to cause birth defects. One enantiomer caused the desirable sedative effects, while the other, unavoidably[21] present in equal quantities, caused birth defects.[22]
The herbicide mecoprop is a racemic mixture, with the (R)-(+)-enantiomer ("Mecoprop-P", "Duplosan KV") possessing the herbicidal activity.[23]
Another example is the antidepressant drugs escitalopram and citalopram. Citalopram is a racemate [1:1 mixture of (S)-citalopram and (R)-citalopram]; escitalopram [(S)-citalopram] is a pure enantiomer. The dosages for escitalopram are typically 1/2 of those for citalopram. Here, (S)-citalopram is called a chiral switch of Citalopram.
Enantioselective preparations
There are two main strategies for the preparation of enantiopure compounds. The first is known as chiral resolution. This method involves preparing the compound in racemic form, and separating it into its isomers. In his pioneering work, Louis Pasteur was able to isolate the isomers of tartaric acid because they crystallize from solution as crystals each with a different symmetry. A less common method is by enantiomer self-disproportionation.
The second strategy is asymmetric synthesis: the use of various techniques to prepare the desired compound in high enantiomeric excess. Techniques encompassed include the use of chiral starting materials (chiral pool synthesis), the use of chiral auxiliaries and chiral catalysts, and the application of asymmetric induction. The use of enzymes (biocatalysis) may also produce the desired compound.
A third strategy is Enantioconvergent synthesis, the synthesis of one enantiomer from a racemic precursor, utilizing both enantiomers. By making use of a chiral catalyist, both enantiomers of the reactant result in a single enantiomer of product.[24]
Enantiomers may not be isolable if there is an accessible pathway for racemization (interconversion between enantiomorphs to yield a racemic mixture) at a given temperature and timescale. For example, amines with three distinct substituents are chiral, but with few exceptions (e.g. substituted N-chloroaziridines), they rapidly undergo "umbrella inversion" at room temperature, leading to racemization. If the racemization is fast enough, the molecule can often be treated as an achiral, averaged structure.
Enantiopure medications
Advances in industrial chemical processes have made it economic for pharmaceutical manufacturers to take drugs that were originally marketed as a racemic mixture and market the individual enantiomers. This strategy of marketing of a chiral specific drug from an already approved and existing racemic drug is normally done for better therapeutic efficacy. This kind of switching from a racemic drug to an enantiopure drug is called a chiral switch and the process is called chiral switching. In some cases, the enantiomers have genuinely different effects. An interesting case is that of Propoxyphene. The enantiomeric pair of propoxyphene is separately sold by Eli Lilly and company. One of the partner is dextropropoxyphene, an analgesic agent (Darvon) and the other is called levopropoxyphene, an effective antitussive (Novrad).[25][26] It is interesting to note that the trade names of the drugs, DARVON and NOVRAD, also reflect the chemical mirror-image relationship. In other cases, there may be no clinical benefit to the patient. In some jurisdictions, single-enantiomer drugs are separately patentable from the racemic mixture.[27] It is possible that only one of the enantiomers is active. Or, it may be that both are active, in which case separating the mixture has no objective benefits, but extends the drug's patentability.[28]
Parity violation
For all intents and purposes, each enantiomer in a pair has the same energy. However, theoretical physics predicts that due to parity violation of the weak nuclear force (the only force in nature that can "tell left from right"), there is actually a minute difference in energy between enantiomers (on the order of 10−12 eV or 10−10 kJ/mol or less) due to the weak neutral current mechanism. This difference in energy is far smaller than energy changes caused by even small changes in molecular conformation, and far too small to measure by current technology, and is therefore chemically inconsequential.[15][29][30] In the sense used by particle physicists, the "true" enantiomer of a molecule, which has exactly the same mass-energy content as the original molecule, is a mirror-image that is also built from antimatter (antiprotons, antineutrons, and positrons).[15] Throughout this article, "enantiomer" is used only in the chemical sense of compounds of ordinary matter that are not superposable on their mirror image.
Quasi-enantiomers
Quasi-enantiomers are molecular species that are not strictly enantiomers, but behave as if they are. Quasi-enantiomers have applications in parallel kinetic resolution.[31]
See also
- Chiral switch
- Crystal system
- Enantiopure drug
- Atropisomer
- Chirotechnology
- Chirality (physics)
- Diastereomer
- Dynamic stereochemistry
- Epimer
- Molecular symmetry
- Stereochemistry
- Stereocenter
References
- "enantiomer". Lexico UK English Dictionary. Oxford University Press. Archived from the original on 2021-01-10.
- IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "optical isomers". doi:10.1351/goldbook.O04308
- IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "antipode". doi:10.1351/goldbook.A00403
- IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "optical antipodes". doi:10.1351/goldbook.O04304
- IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "enantiomer". doi:10.1351/goldbook.E02069
- IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "enantiomorph". doi:10.1351/goldbook.E02079
- IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "enantiomerically pure (enantiopure)". doi:10.1351/goldbook.E02072
- IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "racemate". doi:10.1351/goldbook.R05025
- Ariens, E. J. (1984). "Stereochemistry, a basis for sophisticated nonsense in pharmacokinetics and clinical pharmacology". European Journal of Clinical Pharmacology. 26 (6): 663–668. doi:10.1007/bf00541922. ISSN 0031-6970. PMID 6092093. S2CID 30916093.
- Ariëns, Everardus J. (1986). "Stereochemistry: A source of problems in medicinal chemistry". Medicinal Research Reviews. 6 (4): 451–466. doi:10.1002/med.2610060404. ISSN 0198-6325. PMID 3534485. S2CID 36115871.
- "P-101.8.1 Inversion of configuration" (PDF), IUPAC Provisional Recommendations—Preferred IUPAC Names, p. 48
- Karras, Manfred (2018). "Synthesis of Enantiomerically Pure Helical Aromatics Such As NHC Ligands and Their Use in Asymmetric Catalysis (PhD). Charles University. Retrieved 6 August 2021.
- IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "chirality centre". doi:10.1351/goldbook.C01060
- Wade, LeRoy G. (2006). "Precision in Stereochemical Terminology". J. Chem. Ed. American Chemical Society (ACS). 83 (12): 1793. doi:10.1021/ed083p1793. ISSN 0021-9584.
- Eliel, Ernest L.; Wilen, Samuel H.; Mander, Lewis N. (1994). Stereochemistry of organic compounds. New York: Wiley. ISBN 0471016705. OCLC 27642721.
- Clayden, Jonathan; Greeves, Nick; Warren, Stuart G. (2012). Organic chemistry. Oxford: Oxford University Press. ISBN 978-0-19-927029-3. OCLC 761379371.
- IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "asymmetric centre". doi:10.1351/goldbook.A00480
- Clark, Andrew; Kitson, Russell R. A.; Mistry, Nimesh; Taylor, Paul; Taylor, Matthew; Lloyd, Michael; Akamune, Caroline (2021). Introduction to stereochemistry. Cambridge, UK. ISBN 978-1-78801-315-4. OCLC 1180250839.
- IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "stereogenic unit (stereogen/stereoelement)". doi:10.1351/goldbook.S05980
- Mislow, Kurt; Siegel, Jay (1984). "Stereoisomerism and local chirality". J. Am. Chem. Soc. 106 (11): 3319–3328. doi:10.1021/ja00323a043. ISSN 0002-7863.
- Knoche, B; Blaschke, G (1994). "Investigations on the in vitro racemization of thalidomide by high-performance liquid chromatography". Journal of Chromatography A. Elsevier. 666 (1–2): 235–240. doi:10.1016/0021-9673(94)80385-4.
- Voet, Donald; Voet, Judith G.; Pratt, Charlotte W. (2006). Fundamentals of Biochemistry. p. 89. ISBN 0-471-21495-7.
- G. Smith; C. H. L. Kennard; A. H. White; P. G. Hodgson (April 1980). "(±)-2-(4-Chloro-2-methylphenoxy)propionic acid (mecoprop)". Acta Crystallogr. B. 36 (4): 992–994. doi:10.1107/S0567740880005134.
- Mohr, J.T.; Moore, J.T.; Stoltz, B.M. (2016). "Enantioconvergent catalysis". Beilstein J. Org. Chem. 12: 2038–2045. doi:10.3762/bjoc.12.192. PMC 5082454. PMID 27829909. Retrieved 4 August 2021.
- Drayer, Dennis E (1986). "Pharmacodynamic and pharmacokinetic differences between drug enantiomers in humans: An overview". Clinical Pharmacology and Therapeutics. 40 (2): 125–133. doi:10.1038/clpt.1986.150. ISSN 0009-9236. PMID 3731675. S2CID 33537650.
- Ariens, E.J (1989). Chiral Separations by HPLC. Chichester: Ellis Horwwod, Chichester. pp. 31–68.
- "European Medicines Agency - - Sepracor Pharmaceuticals Ltd withdraws its marketing authorisation application for Lunivia (eszopiclone)". www.ema.europa.eu.
- Merrill Goozner (2004). The $800 Million Pill: The Truth Behind the Cost of New Drugs (excerpt). University of California Press. ISBN 0-520-23945-8.
- Albert, Guijarro (2008). The origin of chirality in the molecules of life: a revision from awareness to the current theories and perspectives of this unsolved problem. Yus, Miguel. Cambridge, UK: Royal Society of Chemistry. ISBN 9781847558756. OCLC 319518566.
- Stickler, Benjamin A.; Diekmann, Mira; Berger, Robert; Wang, Daqing (2021-09-14). "Enantiomer Superpositions from Matter-Wave Interference of Chiral Molecules". Physical Review X. 11 (3): 031056. arXiv:2102.06124. doi:10.1103/PhysRevX.11.031056. ISSN 2160-3308. S2CID 231879820.
- G.S. Coumbarides, M. Dingjan, J. Eames, A. Flinn, J. Northen and Y. Yohannes, Tetrahedron Lett. 46 (2005), p. 2897er
External links
 Media related to Enantiomers at Wikimedia Commons
Media related to Enantiomers at Wikimedia Commons- chemwiki:stereoisomerism