Elimination is a type of organic reaction in which a leaving group and vicinal hydrogen are removed, leaving behind a double bond. Like nucleophilic substitution, it can occur via unimolecular or bimolecular mechanisms.
A common example of elimination is the dehydration reaction, which occurs in a unimolecular process:
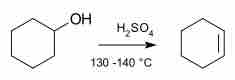
Dehydration of Cyclohexane
Unimolecular Elimination
In unimolecular elimination (E1), a leaving group first detaches itself from a carbon atom, leaving behind a carbocation. This is the slow step that is forced by high temperatures and facilitated by polar sovents, and is exactly the same first step as in an SN1 reaction. A base then deprotonates a carbon adjacent to the carbocation, and the electrons from the previous C-H bond then go to form a C=C bond at the carbocation. The mechanism can be illustrated as:
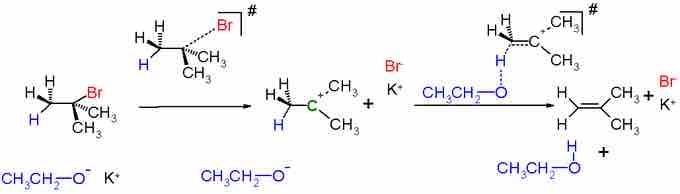
E1 Mechanism
Because their first step is the same and nucleophilicity and basicity often correlate, SN1 and E1 mechanisms will compete. E1 is favored with more substituted carbocations, which are less accessible to nucleophiles. E1 is also favored with larger, stronger bases, although range of usefulness in basicity is limited is the reaction is performed under acidic conditions. Finally, high temperatures favor E1.
Because E1 reactions are performed under thermodynamic control (at high temperature), the most thermodynamically stable product is favored. Thus, if there are hydrogen atoms attached to multiple carbon atoms vicinal to the carbocation, the double bond will be formed between the former carbocation and the most substituted vicinal carbon.
Also as a result of E1 being under thermodynamic control, production of the e-alkene isomer, which experiences lesser steric hindrance than the z-isomer, will be favored.
Bimolecular Elimination
In bimolecular elimination (E2), a base deprotonates a carbon vicinal to a leaving group, and the electrons from the C-H bond form a double bond with the adjacent carbon, displacing the leaving group. The mechanism can be illustrated as:
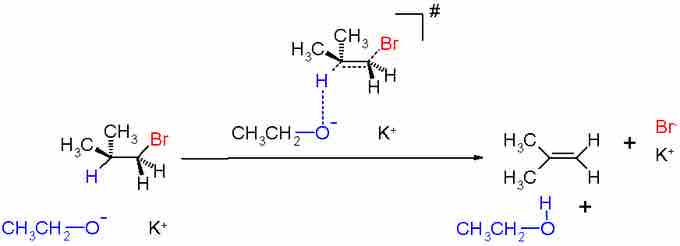
E2 Mechanism
All electron transfers in an E2 reaction occur by a concerted, one-step mechanism, which bears some similarity to SN2. As in SN2, the leaving group (LG) is "pushed" away by electrons that access the C-LG antibonding orbital. In the case of E2, at the time of deprotonation the hydrogen must be antiperiplanar to the leaving group. That way, the electrons from the C-H bond can easily fall into the antibonding C-LG orbital, which is found 180 degrees from the C-LG bonding orbital.
E2 competes with SN2, and as with E1 is increasingly favored with increasing base size and substitution at the carbon to which the leaving group is attached. A stronger base is required than in E1, because in E2, the hydrogen is less acidic at the point of removal (there is no vicinal carbocation). Examples of useful E2 bases include the diisopropylamide and tert-butoxide anions. Hydride is also very useful, because it resembles a point-charge and is not polarizable enough to operate as a nucleophile.
As with E1, E2 favors production of the most thermodynamically stable product, so the most substituted double bond possible will be formed, and if possible the e-alkene isomer will be favored.