Stereoisomers, or spatial isomers, are specific types of isomers. Stereoisomers of a compound have not only the same chemical formula as one another (as do all isomers), but the same bond connectivity. What differs between stereoisomers of a compound is the spatial arrangement of atoms. Stereoisomers can be divided into enantiomers, diastereomers and meso compounds.
Enantiomers
Enantiomers are nonsuperimposable mirror images of one another. A simple non-chemical example is a pair of hands. If you hold your left hand fixed and rotate your right hand, you will never be able to superimpose one upon the other. They are mirror images. The adjective used to describe such structures is "chiral. "
In chemistry, chirality is commonly observed in both coordination and organic compounds. In organic chemistry, chirality is almost exclusively a topic concerned with tetrahedral (SP3-hybridized) carbon atoms. When a carbon atom is bonded to four different substituents, it is chiral. Consider lactic acid, for example:
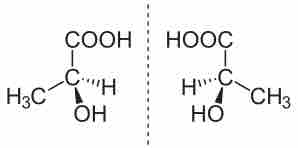
Enantiomers of Lactic Acid
S-Lactic acid and R-Lactic acid are depicted on the left and right, respectively. They share the same formula and bond connectivity, but are nonsuperimposable mirror images of one another.
The compound on the left is known as the S-enantiomer; that on the right is the R. Both compounds have exactly the same chemical and physical properties, except for the direction in which each rotates polarized light (which will be opposites). Determining R vs S can be done by rotating the molecule such that the smallest atom attached to the stereocenter is projected away from the observer (shown by a dashed line) and considering the orientation of the three other substituents. The exact rules for nomenclature will be discussed in another atom.
Although nearly all chiral organic compounds have stereocenters at SP3 carbon centers, there are other possibilities. Quaternary ammonium ions, for example, are chiral if substituted with four different carbon substituents.
Diastereomers
Diastereomers are stereoisomers that are not enantiomers. When multiple stereoisomers of the same compound have different configurations of one or more (but not all) of the related stereoisomers, they are not mirror images.
For example, consider R-Threose:
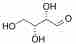
R-Threose
and R-Erythrose:
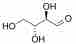
R-Erythrose
Both have the same composition and connectivity. They are not mirror images, however, and are thus diastereomers. A good way to identify diastereomers is to compare stereocenters. In R-Threose and R-Erythrose, the hydroxyl at the 3 carbon points away. The hydroxyl at the 2 carbon differs between them, thus confirming that they are neither the same nor mirror images.
Meso Compounds
Some stereoisomers are not optically active and are actually exactly the same molecule. This occurs when multiple stereocenters "cancel" one another, resulting in symmetry that makes the "different" molecules superimposable. Consider 1,2-dimethylpropane:
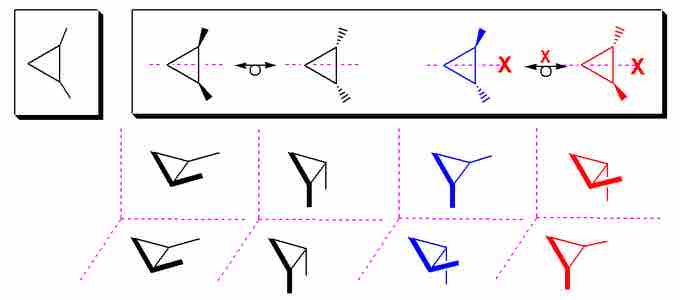
Stereoisomers of 1,2-Dimethylpropane
The two stereoisomers to the left ((R, S) and (S,R)) are identical, and thus are considered meso. The (R, R) and (S, S) isomers are enantiomers.