Respirator Trusted-Source Information
Section 2: Use of NIOSH-Approved Respirators
Information sources on how to implement the appropriate use of respirators in the workplace. This section also contains a listing of recently revoked approvals as well as related User Notices.
Use of NIOSH-Approved Respirators
ADVISORY: Watch for respirators misrepresented as NIOSH-approved! See the misrepresentation section.
Respirator Fit Testing
Respirator Safety
OSHA Respirator Safety Video (click on link and scroll down page to training)
Respirator Safety. Donning (Putting on) and Doffing (Taking off) and User Seal Checks. U.S. Department of Labor Video, (2009, December 16). This video is available in English and Spanish and is available for downloading.
OSHA Voluntary Use of Respirators video
OSHA Respiratory Protection Training Requirements video
Medical Evaluations for Workers Who Use Respirators video
Respirator User Notices
Buyer Beware
Respirator Awareness: Your Health May Depend On It
DHHS (NIOSH) Publication No. 2013-138 (June 2013) Español
One of the occupational hazards in the healthcare setting is the airborne transmission of certain infectious diseases. The potential of exposure is not limited to physicians, nurses, and support personnel in direct patient care. It extends to those delivering food, cleaning patient rooms, and performing maintenance. Anyone working in areas with patients infected with airborne-transmissible diseases is potentially at risk.
OSHA Counterfeit and Altered Respirators: The Importance of NIOSH Certification video
Misrepresentation of NIOSH Approval
There have been reports of products using promotional materials that infer or cite NIOSH approval. Some of these products may look very similar to NIOSH-approved respirators. One example is a product that has taken N95 respirators and added decorative fabric for fashionable effects, therefore voiding the approval (http://www.flufashion.net). Another example is a surgical mask being advertised as a NIOSH-approved N95 respirator. NIOSH purchased the product, tested it and found it to have over 80% leakage. Several instances of packaging that falsely uses terms like “NIOSH-approved”, and “NIOSH N95” have been brought to our attention. Though it is often difficult to be able to tell from first glance if a respirator is truly NIOSH-certified, the most reliable marking to look for is the NIOSH TC# printed both on the box as well as the product itself (TC# xxx-xxxx). The product can be verified by checking the TC number on the NIOSH website.
Example of typical markings on filtering facepiece respirators.
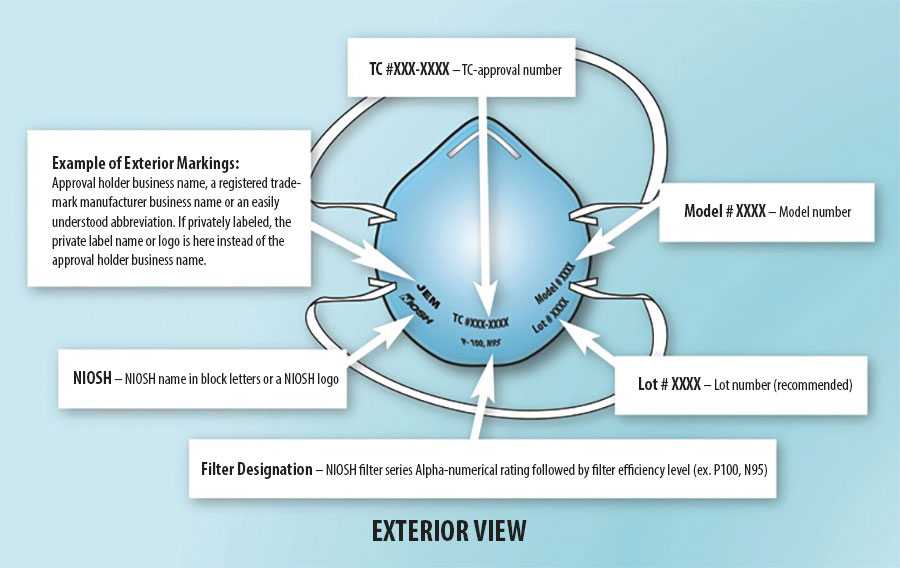
Counterfeit Respirators
When NIOSH becomes aware of counterfeit respirators or those misrepresenting NIOSH approval on the market, we will post them here to alert users, purchasers, and manufacturers.
April 28, 2017 – Counterfeit Respirators or Misrepresentation of NIOSH Approval

Figure 1 is an example of a counterfeit N95 Respirator that was brought to NIOSH’s attention. While the TC number and private label holder are valid, this unapproved unit can be identified by the misspelling of NIOSH on the front of the respirator.


Figures 2 and 3 are examples of counterfeit respirators. These respirators are being sold as if they are NIOSH-approved even though the manufacturer, Zubi-Ola, is not listed as a NIOSH approval holder or a private label holder.
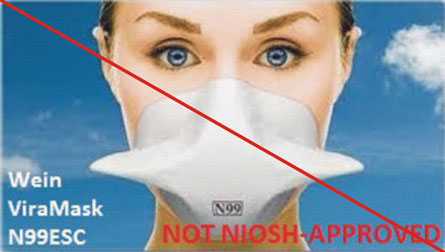
Figure 4 is an example of misrepresentation of the NIOSH approval. All approvals for Wein Products (WPI) were rescinded in 2011. However, the manufacturer’s website continues to state the ViraMask N99ESC is certified by NIOSH. View the user notice announcing the rescission.
Check the respirator approval markings (graphic below) or the Certified Equipment List to verify your respirator is NIOSH-approved. Additional information is available on the NIOSH Trusted Source page.
Figure 5 shows a NIOSH-approved filtering facepiece. Figure 6 shows the same type of filtering facepiece with a decorative design. Respirators featuring such designs are not NIOSH-approved.
Figures 5 and 6:


The earloop masks below have been advertised as N95 NIOSH-approved on eBay and elsewhere on the internet. NIOSH does not approve surgical masks, only respirators. The masks pictured below are not NIOSH-approved.
Figures 7 and 8:


The masks below are being sold as N95. NPPTL purchased and tested these masks and found them to have over 80% leakage. These masks are not NIOSH-approved.
Figures 9 and 10:


The box for this product indicates NIOSH approval. However, NIOSH does not approve surgical masks.

Rescinded Respirator Approvals
Periodically, respirator approvals are rescinded either at the request of the approval holder, or revoked by NIOSH for cause. Depending upon the reason for the rescission, a respirator User Notice may be issued. With all rescissions, the approval number is no longer listed in the Certified Equipment List (CEL), or on any of the NIOSH web pages that list approved respirators. View user notices issued prior to 2011.
The validity of a respirator approval should be verified on NIOSH Certified Equipment List.
Related User Notices
Respirator User Notices are issued on a variety of topics related to NIOSH-approved respirators. The notices listed immediately below contain information that may help the user understand important issues related to respirator approval.
July 17, 2012 – Misleading Representation of the Use of Adhesive Seals as NIOSH-Approved
August 23, 2010 – Voluntary Stop Sale by Emany Consulting due to Mislabeled Packaging [PDF – 36 KB]
August 23, 2010 – Voluntary Stop Sale by Emany Consulting due to Question Regarding Protection Level [PDF – 34 KB]
January 16, 2009 – NIOSH Respirator Certification [PDF – 81.1 KB]
May 04, 2007 – Respirator User Notice, Use of Replacement and Spare Parts [PDF – 39 KB]
March 17, 2006 – Respirator User Notice, Meaning of NIOSH Approval [PDF – 28 KB]
Particulate Respirator User Notices
The notices listed below pertain to topics related to particulate respirators:
March 7, 2013 – Voluntary Rescission of Honeywell International Inc. (North Safety Products) Certificates of Approval TC-84A-3722, TC-84A-4240, and TC-84A-4251
July 31, 2012 – Voluntary Recission of Thea-Tex Healthcare Pvt. Ltd. Certificate of Approval TC-84A-5296
July 25, 2012 – Voluntary Rescission of PnTD Corporation Certificates of Approval TC-84A-5494 and TC-84A-5630
April 30, 2012 – Voluntary Rescission of Jiangyin Changhua Industrial Manufacturing Co., Ltd. Certificate of Approval TC-84A-5230
December 21, 2011 – Voluntary Rescission of FINEtech Company, Ltd. Certificate of Approval TC-84A-5461, Effective December 15, 2011
November 8, 2011 – Voluntary Rescission of Aswan International Corporation Certificate of Approvals TC-84A-4563 and TC-84A-4632, Effective November 7, 2011
October 21, 2011 – Voluntary Recission of Qiaohong Optical Industry Co., Ltd. Certificate of Approvals TC-84A-4532 and TC-84A-4630, Effective October 21, 2011
June 23, 2011 – Voluntary Rescission of Emany Consulting Certificate of Approvals TC-84A-4177, TC-84A-4191, and TC-84A-4239, Effective June 23, 2011
May 18, 2011 – Voluntary Rescission of Jinfuyu Industrial Co., Ltd., Certificate of Approval TC-84A-4113, Effective May 18, 2011
April 20, 2010 – Voluntary Rescission of Champak Enterprise Co. Ltd Certificate of Approvals, TC-84A-4363 and TC-84A-4364, Effective April 20, 2010 [PDF – 34 KB]
December 16, 2009 – Voluntary Rescission of all Respirator Certificates of Approval Issued to Guangzhou Weini Technology & Development Co., Ltd. (GWT) Effective December 16, 2009. [PDF – 26 KB]
August 30, 2009 – Revocation of all Respirator Certificates of Approval Issued to Dual Safe Life (DSL) Effective August 30, 2009. [PDF – 871 KB]
May 5, 2009 – Misleading Representation of the DuraMax PRO EN149 FPP1 Dust Mask as NIOSH Approved N95 [PDF – 105 KB]
August 21, 2008 – Misleading Representation of the Mainstays Projects RSP2MS Respirator as NIOSH-Approved N95 [PDF – 614 KB]
September 28, 2006 – Misleading Representation of the Mainstays Projects Respirator as a NIOSH-Approved N95 Respirator [PDF – 77 KB]
June 30, 2006 – Corrected Respirator User Notice – NIOSH voiding Crew, Inc Certificates of Approval [PDF – 25 KB]
- Page last reviewed: October 12, 2017
- Page last updated: May 1, 2017
- Content source:
- National Institute for Occupational Safety and Health National Personal Protective Technology Laboratory


 ShareCompartir
ShareCompartir