Respirator User Notices Issued by NIOSH
User Notice – Notices are provided to inform users of a condition or risk that may exist with a NIOSH-approved respirator.
NIOSH has reviewed and concurs with the facts in the user notice as of the date indicated on the NIOSH website.
Disclaimer: Links to non-Federal organizations do not constitute an endorsement of these organizations, their products, or programs by NIOSH and none should be inferred. NIOSH is not responsible for the content of the individual organization web pages found at these links. They are provided solely as a service to our users. A NIOSH certificate of approval is not an endorsement of the respirator by NIOSH. Such endorsements are not to be stated or implied by manufacturers in advertisements or other publicity. NIOSH certification represents that the equipment has met the requirements of Title 42, Code of Federal Regulations (CFR) Part 84.
Voluntary Rescissions of Respirator Certificates of Approval as requested by Manufacturer
With all rescissions, the approval number is no longer listed in the Certified Equipment List (CEL), or on any of the NIOSH web pages that list approved respirators.
- SAS Safety Corporation – Voluntary Rescission – October 3, 2016
- Global Safety First – Voluntary Rescission – November 10, 2014
- Handan Hengyong Protective and Clean Products Co., Ltd – Voluntary Rescission – September 8, 2014
- priMED Medical Products Inc. – Voluntary Rescission – August 6, 2014
- Fido Mask Company – Voluntary Rescission – August 3, 2014
- Suzhou Fangtian Industries, Ltd. – July 23, 2014
- Filligent (HK) Ltd. – Voluntary Rescission – May 19, 2014
NIOSH-Issued Notices
- October 3, 2016 – Voluntary Rescission of SAS Safety Corporation, Certificates of Approval for TC 19C-0424, TC-19C-0425, TC-19C-0426, TC-19C-0427, TC-19C-0466, TC-23C-2305, TC-23C-2802, TC-23C-2893, TC-84A-4354, TC-84A-5195, TC-84A-5351, TC-84A-5412, TC-84A-6665, TC-84A-6753, TC-84A-6768, TC-84A-7450, TC-84A-7451, TC-84A-7452, TC-84A-7526, TC-84A-7527, TC-84A-7620, TC-84A-7634, and TC 84A 7640
- September 22, 2016 – Revocation of all Respirator Certificates of Approval Issued to NITTA Corp. S.A., Effective September 22, 2016
- July 31, 2014 – Update – Use Restrictions Lifted from NIOSH CBRN Respirator Approvals
- July 23, 2014 – Voluntary Rescission of Suzhou Fangtian Industries Co., Ltd. Certificates of Approval for TC-84A-5481, TC-84A-5482, TC-84A-5483, TC-84A-5531, and TC-84A-5532
- June 2, 2014 – Mine Operator Annual Testing of Self-Contained Self-Rescuer (SCSR) Units
- May 19, 2014 – Voluntary Rescission of Filligent (HK) Ltd. Certificate of Approval TC-84A-6252
- February 27, 2014 – Use of After Market Component Parts
Archive of NIOSH-Issued Notices Prior to 2014
Respirator User Notices Issued by Manufacturers
Counterfeit Respirators / Misrepresentation of NIOSH-Approval
When NIOSH becomes aware of counterfeit respirators or those misrepresenting NIOSH approval on the market, we will post them here to alert users, purchasers, and manufacturers.
April 28, 2017 – Counterfeit Respirators or Misrepresentation of NIOSH Approval

Figure 1 is an example of a counterfeit N95 Respirator that was brought to NIOSH’s attention. While the TC number and private label holder are valid, this unapproved unit can be identified by the misspelling of NIOSH on the front of the respirator.


Figures 2 and 3 are examples of counterfeit respirators. These respirators are being sold as if they are NIOSH-approved even though the manufacturer, Zubi-Ola, is not listed as a NIOSH approval holder or a private label holder.
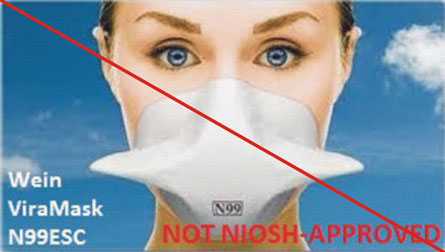
Figure 4 is an example of misrepresentation of the NIOSH-approval. All approvals for Wein Products (WPI) were rescinded in 2011. However, the manufacturer’s website continues to state the ViraMask N99ESC is certified by NIOSH. View the user notice announcing the rescission.
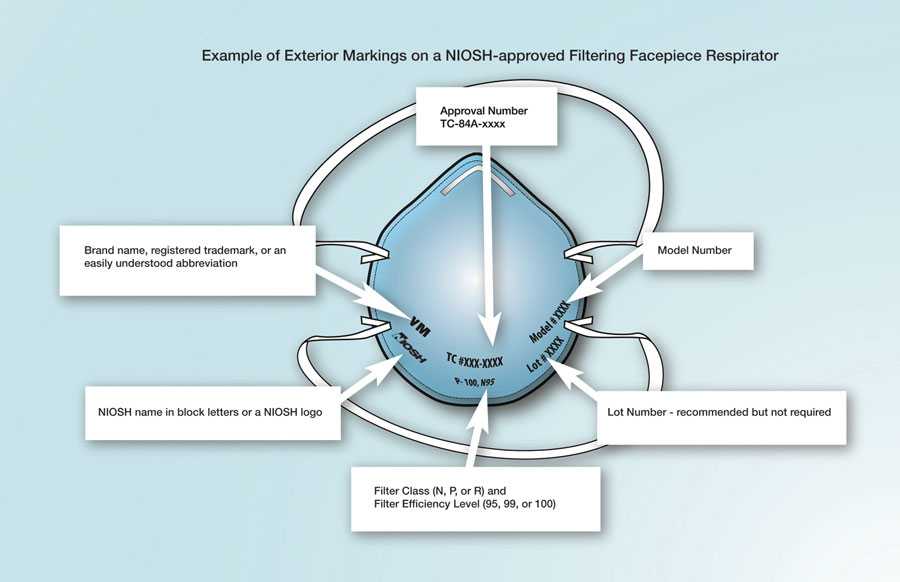
Check the respirator approval markings (graphic below) or the Certified Equipment List to verify your respirator is NIOSH-approved. Additional information is available on the NIOSH Trusted Source page.
Example of the Correct Exterior Markings on a NIOSH-Approved Filtering Facepiece Respirator
- Page last reviewed: October 11, 2017
- Page last updated: May 1, 2017
- Content source:
- National Institute for Occupational Safety and Health National Personal Protective Technology Laboratory


 ShareCompartir
ShareCompartir