Diphtheria, Tetanus, and Pertussis Vaccine Safety
On This Page
Diphtheria, Tetanus, and Pertussis Diseases and How to Protect Against Them
Diphtheria, tetanus, and pertussis are three bacterial diseases that can be vaccinated against with a single shot.
- Diphtheria causes a thick covering in the back of the throat. It can lead to breathing problems, paralysis, heart failure, and even death.
- Tetanus (lockjaw) is a serious disease that causes painful tightening of the muscles, usually all over the body. It can lead to “locking” of the jaw so the victim cannot open their mouth or swallow. Tetanus leads to death in about 1 in 10 cases.
- Whooping cough — known medically as pertussis — is a highly contagious respiratory tract infection. Although it initially resembles an ordinary cold, whooping cough may eventually turn more serious, particularly in infants.
You can protect against these diseases with safe, effective vaccination. These vaccines include:
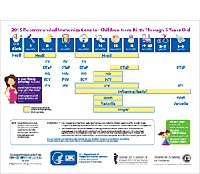
- DTaP (diphtheria, tetanus, and acellular pertussis), which is given to children
- Td (adult tetanus and diphtheria), which is given to adolescents and adults
- Tdap (combined tetanus, diphtheria and pertussis), which is given to adolescents and adults
Diphtheria, Tetanus, and Pertussis Vaccine Side Effects
DTaP, Td, and Tdap vaccines are very safe and effective at preventing diphtheria, tetanus, and pertussis. Vaccines, like any medicine, can have side effects. The most common side effects are usually mild and go away on their own.
Common Side Effects of DTap Vaccines
- Soreness or swelling where the shot was given
- Fever
- Irritability
- Feeling tired
- Loss of appetite
- Vomiting
Common Side Effects of Td Vaccines
- Pain, redness, or swelling where the shot was given
- Mild fever
Common Side Effects of Tdap Vaccines
- Pain, redness, or swelling where the shot was given
- Mild fever
- Headache
- Feeling tired
- Nausea, vomiting, diarrhea, stomach ache
On very rare occasions, severe (anaphylactic) allergic reactions may occur after vaccination, including hives, swelling of the face and throat, difficulty breathing, a fast heartbeat, dizziness, and weakness.
Available Diphtheria, Tetanus, and Pertussis Vaccines
DTaP (for younger children):
- Daptacel [PDF – 336 KB]: The Food and Drug Administration (FDA) approved this vaccine in 2002. It is approved for use in children who are 6 weeks through 6 years of age to protect against diphtheria, tetanus, and pertussis.
- Infanrix [PDF – 210 KB]: FDA approved this vaccine in 1997. It is approved for use in children who are 6 weeks through 7 years of age to protect against diphtheria, tetanus, and pertussis.
- Kinrix [PDF – 165 KB]: FDA approved this vaccine in 2008. It is approved for use in children who are 4 to 6 years of age to protect against diphtheria, tetanus, pertussis, and polio.
- Pediarix [PDF – 242 KB]: FDA approved this vaccine in 2002. It is approved for use in children who are 6 weeks through 6 years of age to protect against diphtheria, tetanus, pertussis, polio, and hepatitis B.
- Pentacel [PDF – 325 KB]: FDA approved this vaccine in 2008. It is approved for use in children who are 6 weeks through 18 months of age to protect against diphtheria, tetanus, pertussis, Haemophilus Influenzae type B (Hib), and polio.
- Quaracel [PDF – 178 KB]: FDA approved this vaccine in 2015. It is approved for use in children who are 4 through 6 years of age to protect against diphtheria, tetanus, pertussis, and polio.
- There is also a generic [PDF – 106 KB] vaccine to protect against diphtheria and tetanus in children through 6 years of age. It was approved by FDA in 1978.
Td (for adolescents and adults):
- Decavac [PDF – 276 KB]: FDA approved this vaccine in 1955. It is approved for use in people 7 years of age and older to protect against diphtheria and tetanus.
- Tenivac [PDF – 304 KB]: FDA approved this vaccine in 2003. It is approved for use in people 7 years of age and older to protect against diphtheria and tetanus.
- There is also a generic [PDF – 65 KB] vaccine to protect against diphtheria and tetanus in people 7 years of age and older. It was approved by FDA in 1967.
Tdap (for adolescents and adults):
- Boostrix [PDF – 316 KB]: FDA approved this vaccine in 2005. It is approved for use in people 10 years of age and older to protect against diphtheria, tetanus, and pertussis.
- Adacel [PDF – 245 KB]: FDA approved this vaccine in 2005. It is approved for use in people 10 through 64 years of age to protect against diphtheria, tetanus, and pertussis.
How CDC Monitors Diphtheria, Tetanus, and Pertussis Vaccine Safety
CDC and FDA continuously monitor the safety of vaccines after they are approved. If a problem is found with a vaccine, CDC and FDA will inform health officials, health care providers, and the public.
CDC uses three systems to monitor vaccine safety:
- The Vaccine Adverse Event Reporting System (VAERS): an early warning system that helps CDC and FDA monitor problems following vaccination. Anyone can report possible vaccine side effects to VAERS.
- The Vaccine Safety Datalink (VSD): a collaboration between CDC and nine health care organizations which allows ongoing monitoring and proactive searches of vaccine-related data.
- The Clinical Immunization Safety Assessment (CISA) Project: a partnership between CDC and several medical centers that conducts clinical research on vaccine-associated health risks.
A Closer Look at the Safety Data
DTaP (younger children)
- DTaP studies among VAERS reports found no health concerns related to the vaccine.
- Several studies of DTaP vaccine safety have looked for neurologic problems or seizures after children were vaccinated, and found that there is no increased risk for these concerns.
- DTaP may frequently cause mild injection site reactions. However, severe reactions are rare, and may be less frequent when the vaccine is injected into the leg than into the arm. Reactions happen about as often when DTaP is combined with other vaccines.
Tdap (adolescents and adults)
- Tdap safety studies among VAERS reports have found no safety concerns for the general population, for pregnant women, or for adults over age 65.
- In the VSD, studies have found no association between Tdap vaccination and Guillain-Barre Syndrome or other neurologic disorders. Other studies have found that there is no increased risk for other types of health problems, such as allergies, blood disorders, and chronic illnesses.
- Although injection site reactions are common, studies have found a low rate of severe reactions. These local reactions are unusual even when the vaccine is given at the same time as meningococcal conjugate vaccine (Menactra), or when a person receives several doses of Tdap vaccine over a short time period.
More Resources
DTaP:
- Diphtheria, Tetanus, and Pertussis (DTaP) Vaccine Information Statement
- DTaP Vaccine: Who Should Not Get Vaccinated
Td:
Tdap:
- Tdap (Tetanus, Diphtheria, Pertussis) Vaccine Information Statement
- Tdap Vaccine: Who Should Not Get Vaccinated
General:
- Tetanus (Lockjaw) Vaccination
- Diphtheria Vaccination
- Pertussis (Whooping Cough) Vaccination
- U.S. Vaccine Abbreviations [PDF – 196 KB]
Related Scientific Articles
DTap
Braun MM, Mootrey GT, Salive ME, Chen RT, Ellenberg SS. Infant immunization with acellular pertussis vaccines in the United States: Assessment of the first two years’ data from the Vaccine Adverse Event Reporting System (VAERS). Pediatrics. 2000 Oct;106(4):E51.
Huang WT, Gargiullo PM, Broder KR, Weintraub ES, Iskander JK, et al. Lack of association between acellular pertussis vaccine and seizures in early childhood. Pediatrics. 2010 Aug;126(2):263-9.
Jackson LA, Yu O, Nelson JC, Dominguez C, Peterson D, et al. Injection site and risk of medically attended local reactions to acellular pertussis vaccine. Pediatrics. 2011 Mar;127(3):e581-7.
Moore DL, Le Saux N, Scheifele D, Halperin SA; Members of the Canadian Paediatric Society/Health Canada Immunization Monitoring Program Active (IMPACT). Lack of evidence of encephalopathy related to pertussis vaccine: Active surveillance by IMPACT, Canada, 1993-2002. Pediatr Infect Dis J. 2004;23(6):568-71.
Ray P, Hayward J, Michelson D, Lewis E, Schwalbe J, et al. Encephalopathy after whole-cell pertussis or measles vaccination: Lack of evidence for a causal association in a retrospective case-control study. Pediatr Infect Dis J. 2006 Sep;25(9):768-73.
Zangwill KM, Eriksen E, Lee M, Lee J, Marcy SM, et al. A population-based, postlicensure evaluation of the safety of a combination diphtheria, tetanus, acellular pertussis, hepatitis B, and inactivated poliovirus vaccine in a large managed care organization. Pediatrics. 2008;122(6):e1179-85.
Tdap
Chang S, O’Connor PM, Slade BA, Woo EJ. U.S. Postlicensure safety surveillance for adolescent and adult tetanus, diphtheria and acellular pertussis vaccines: 2005-2007. Vaccine. 2013 Feb 27;31(10):1447-52.
Halperin SA, Sweet L, Baxendale D, Neatby A, Rykers P, et al. How soon after a prior tetanus-diphtheria vaccination can one give adult formulation tetanus-diphtheria-acellular pertussis vaccine? Pediatr Infect Dis J. 2006;25(3):195-200.
Jackson LA, Yu O, Nelson J, Belongia EA, Hambidge SJ, et al. Risk of medically attended local reactions following diphtheria toxoid containing vaccines in adolescents and young adults: A Vaccine Safety Datalink study. Vaccine. 2009 Aug 6;27(36):4912-6.
Klein NP, Hansen J, Lewis E, Lyon L, Nguyen B, et al. Post-marketing safety evaluation of a tetanus toxoid, reduced diphtheria toxoid and 3-component acellular pertussis vaccine administered to a cohort of adolescents in a United States health maintenance organization. Pediatr Infect Dis J. 2010;29(7):613-7.
Moro PL, Yue X, Lewis P, Haber P, Broder K. Adverse events after tetanus toxoid, reduced diphtheria toxoid and acellular pertussis (Tdap) vaccine administered to adults 65 years of age and older reported to the Vaccine Adverse Event Reporting System (VAERS), 2005-2010. Vaccine. 2011 Nov 21;29(50):9404-8
Nordin JD, Yih WK, Kulldorff M, Weintraub E. Tdap and GBS (letter). Vaccine. 2011 Feb 1;29(6):1122.
Talbot EA, Brown KH, Kirkland KB, Baughman AL, Halperin SA, et al. The safety of immunizing with tetanus-diphtheria-acellular pertussis vaccine (Tdap) less than 2 years following previous tetanus vaccination: Experience during a mass vaccination campaign of healthcare personnel during a respiratory illness outbreak. Vaccine. 2010;28(50):8001-7.
Yih WK, Nordin JD, Kulldorff M, Lewis E, Lieu TA, et al. An assessment of the safety of adolescent and adult tetanus-diphtheria-acellular pertussis (Tdap) vaccine, using active surveillance for adverse events in the Vaccine Safety Datalink. Vaccine. 2009 Jul 9;27(32):4257-62.
Zheteyeva YA, Moro PL, Tepper NK, Rasmussen SA, Barash FE, et al. Adverse event reports after tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccines in pregnant women. Am J Obstet Gynecol. 2012;207:59.e1-7.
- Page last reviewed: October 27, 2015
- Page last updated: October 27, 2015
- Content Source:


 ShareCompartir
ShareCompartir