Methylergometrine
 | |
| Names | |
|---|---|
| Trade names | Methergine, Ergotrate, others |
| Other names | Methylergometrine maleate, methylergonovine,[1] methylergobasin,[1] d-lysergic acid 1-butanolamide |
IUPAC name
| |
| Clinical data | |
| Main uses | Post partum bleeding[1] |
| Pregnancy category |
|
| Routes of use | Injection into a muscle or vein, by mouth[2] |
| Defined daily dose | 0.2 mg[3] |
| External links | |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a601077 |
| Legal | |
| Legal status |
|
| Pharmacokinetics | |
| Metabolism | Liver |
| Elimination half-life | 30–120 min |
| Excretion | Mostly bile |
| Chemical and physical data | |
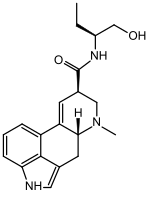
| Formula | C20H25N3O2 |
| Molar mass | 339.439 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 172 °C (342 °F) |
| Solubility in water | insoluble mg/mL (20 °C) |
SMILES
| |
InChI
| |
Methylergometrine, sold under the brand name Methergine among others, is a medication used to treat post partum bleeding due to poor uterine contraction.[2] It is less preferred to oxytocin for this use.[1] It has also been used to check if people have angina due to coronary vasospasms.[2] It is used by injection into a muscle, by mouth, or less commonly by injection into a vein.[1][2]
Common side effects include high blood pressure, seizures, headache, nausea, and low blood pressure.[2] Other side effects may include ringing in the ears, numbness, confusion, and chest pain.[1] Caution is advised in those who have liver problems, kidney problems, or poor blood flow.[1] Safety in pregnancy or breastfeeding is unclear and thus in these situations use is not advised.[1][2] It is in the family of medicines known as oxytocics and ergot alkaloids.[2][4]
Methylergometrine is derived from ergometrine which was initially isolated from the fungus Claviceps purpurea in 1935.[5] It is on the World Health Organization's List of Essential Medicines as an alternative to ergometrine.[6] In the United States it is available as a generic medication and costs about 68 USD for 6 tablets of 0.2 mg as of 2020.[7] In the developing world this amount costs about 0.13 USD as of 2015 while the injectable form is about 0.18 USD per dose.[8][9]
Medical uses
Post partum bleeding
Methylergometrine is a smooth muscle constrictor that mostly acts on the uterus. It is most commonly used to prevent or control excessive bleeding following childbirth and spontaneous or elective abortion, and also to aid in expulsion of retained products of conception after a missed abortion (miscarriage in which all or part of the fetus remains in the uterus) and to help deliver the placenta after childbirth. It is available as tablets or injection (IM or IV) or in liquid form to be taken by mouth.[10][11][12] The intravenous route is less preferred due to side effects.[2]
Migraine
Methylergometrine is sometimes used for both prevention[13] and acute treatment of migraine.[14]
Dosage
The defined daily dose is 0.2 mg by mouth or by injection.[3] For post partum bleeding this is given as intramuscular injections of 0.2 mg every two to four hours to a maximum dose of 1 gram.[1]
Contraindications
Methylergometrine is contraindicated in people with hypertension including pre-eclampsia.[10] It is also contraindicated in people who are HIV positive taking protease inhibitors, delavirdine and efavirenz (which is also an agonist at the 5HT2A-mGlu2 receptor protomer and increases the chances of experiencing hallucinations during methylergometrine therapy).[15]
Side effects
Side effects include:[10]
- Cholinergic effects such as nausea, vomiting, and diarrhea
- Dizziness
- Pulmonary hypertension
- Coronary artery vasoconstriction
- Severe systemic hypertension (especially in patients with pre-eclampsia)
- Convulsions
In excessive doses, methylergometrine can also lead to cramping, respiratory depression and coma.[10]
Interactions
Methylergometrine likely interacts with drugs that inhibit the liver enzyme CYP3A4, such as azole antifungals, macrolide antibiotics and many HIV drugs. It can also increase constriction of blood vessels caused by sympathomimetic drugs and other ergot alkaloids.[10]
Mechanism of action
Methylergometrine is a partial agonist/antagonist on serotonergic, dopaminergic and alpha-adrenergic receptors. Its specific binding and activation pattern on these receptors leads to a highly, if not completely, specific contraction of smooth uterus muscle via 5-HT2A serotonin receptors,[16] while blood vessels are affected to a lesser extent compared to other ergot alkaloids.[10]
References
- 1 2 3 4 5 6 7 8 9 "METHYLERGOMETRINE injectable - Essential drugs". medicalguidelines.msf.org. Archived from the original on 29 August 2021. Retrieved 2 September 2020.
- 1 2 3 4 5 6 7 8 "Methylergonovine Maleate Monograph for Professionals". Drugs.com. Archived from the original on 20 September 2016. Retrieved 2 September 2020.
- 1 2 "WHOCC - ATC/DDD Index". www.whocc.no. Archived from the original on 1 December 2020. Retrieved 2 September 2020.
- ↑ Clinical Pharmacology During Pregnancy. Academic Press. 2012. p. 310. ISBN 978-0-12-386008-8. Archived from the original on 2021-08-29. Retrieved 2020-09-02.
- ↑ Briggs, Gerald G.; Nageotte, Michael (2009). Diseases, Complications, and Drug Therapy in Obstetrics: A Guide for Clinicians. ASHP. p. 264. ISBN 978-1-58528-337-8. Archived from the original on 2021-08-29. Retrieved 2020-09-02.
- ↑ World Health Organization (2023). The selection and use of essential medicines 2023: web annex A: World Health Organization model list of essential medicines: 23rd list (2023). Geneva: World Health Organization. hdl:10665/371090. WHO/MHP/HPS/EML/2023.02.
- ↑ "Methergine Prices and Methergine Coupons". GoodRx. Archived from the original on 16 August 2016. Retrieved 30 October 2020.
- ↑ "Single Drug Information – International Medical Products Price Guide". Archived from the original on 28 August 2021. Retrieved 30 October 2020.
- ↑ "Single Drug Information – International Medical Products Price Guide". Archived from the original on 28 August 2021. Retrieved 30 October 2020.
- 1 2 3 4 5 6 Jasek W, ed. (2007). Austria-Codex (in German) (62nd ed.). Vienna: Österreichischer Apothekerverlag. pp. 5193–5. ISBN 978-3-85200-181-4.
{{cite book}}: CS1 maint: unrecognized language (link) - ↑ Mutschler, Ernst; Schäfer-Korting, Monika (2001). Arzneimittelwirkungen (in German) (8th ed.). Stuttgart: Wissenschaftliche Verlagsgesellschaft. p. 447. ISBN 3-8047-1763-2.
{{cite book}}: CS1 maint: unrecognized language (link) - ↑ "Methergin". Fachinformation des Arzneimittel-Kompendium der Schweiz (in German).
{{cite book}}: CS1 maint: unrecognized language (link) CS1 maint: url-status (link) - ↑ Koehler PJ, Tfelt-Hansen PC (November 2008). "History of methysergide in migraine". Cephalalgia. 28 (11): 1126–35. doi:10.1111/j.1468-2982.2008.01648.x. PMID 18644039.
- ↑ Niño-Maldonado AI, Caballero-García G, Mercado-Bochero W, Rico-Villademoros F, Calandre EP (November 2009). "Efficacy and tolerability of intravenous methylergonovine in migraine female patients attending the emergency department: a pilot open-label study". Head & Face Medicine. 5 (21): 21. doi:10.1186/1746-160X-5-21. PMC 2780385. PMID 19895705.
- ↑ "Methylergonovine Maleate Monograph for Professionals - Drugs.com". drugs.com. Archived from the original on 2016-09-20.
- ↑ Pertz, Heinz; Eich, Eckart (1999). "Ergot alkaloids and their derivatives as ligands for serotoninergic, dopaminergic and adrenergic receptors". In Křen, Vladimír; Cvak, Ladislav (eds.). Ergot: the genus Claviceps. CRC Press. pp. 411–440. ISBN 978-905702375-0.
External links
| Identifiers: |
|
|---|