Trastuzumab emtansine
 | |
| Monoclonal antibody | |
|---|---|
| Type | Whole antibody |
| Source | Humanized (from mouse) |
| Names | |
| Trade names | Kadcyla |
| Other names | Ado-trastuzumab emtansine, trastuzumab-DM1, T-DM1 |
| Clinical data | |
| Drug class | Antibody-drug conjugate[1] |
| Main uses | Breast cancer[2] |
| Side effects | Tiredness, nausea, liver problems, bleeding, muscle pain, constipation[3] |
| Pregnancy category |
|
| Routes of use | Intravenous infusion |
| Typical dose | 3.6 mg/kg[3] |
| External links | |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a613031 |
| Legal | |
| License data | |
| Legal status | |
| Pharmacokinetics | |
| Bioavailability | N/A |
| Protein binding | 93% (in vitro) |
| Metabolism | Liver (CYP3A4/3A5-mediated) |
| Elimination half-life | 4 days |
| Chemical and physical data | |
| Formula | C6448H9948N1720O2012S44·(C47H62ClN4O13S)n |
| Molar mass | 148.5 kg/mol |
Trastuzumab emtansine, sold under the trade name Kadcyla, is a medication used to treat breast cancer.[2] Specifically it is used for HER2 positive which have been treated with other medications.[3] It improved overall survival from 25 months to 31 months.[2] It is given by injection into a vein.[3]
Common side effects include tiredness, nausea, liver problems, bleeding, muscle pain, and constipation.[3] Other side effects may include lung problems, infusion reactions, and neurological problems.[3] Use in pregnancy may harm the baby.[5] It is an antibody-drug conjugate.[1] It consists of the monoclonal antibody trastuzumab that binds to HER2 linked to the cytotoxic agent DM1.[2]
Trastuzumab emtansine was approved for medical use in the United States and Europe in 2013.[3][2] In the United Kingdom the dose for a 70 kg person costs the NHS about £4,300 every 3 weeks as of 2021.[6] This amount in the United States is about 9,400 USD.[7]
Medical use
In the United States, ado-trastuzumab emtansine was approved specifically for treatment of HER2-positive metastatic breast cancer (mBC) in patients who have been treated previously with trastuzumab and a taxane (paclitaxel or docetaxel), and who have already been treated for mBC or developed tumor recurrence within six months of adjuvant therapy.[8][3]
Approval was based on the EMILIA study,[9] a phase III clinical trial that compared trastuzumab emtansine versus capecitabine (Xeloda) plus lapatinib (Tykerb) in 991 people with unresectable, locally advanced or metastatic HER2-positive breast cancer who had previously been treated with trastuzumab and taxane chemotherapy.[9] This trial showed improved progression-free survival in patients treated with trastuzumab emtansine (median 9.6 vs. 6.4 months), along with improved overall survival (median 30.9 vs. 25.1 months) and safety.[10]
Dosage
It is given at a dose of 3.6 mg/kg every 3 weeks (21-day cycle) up to 14 doses.[3]
Side effects
During clinical trials, the most common adverse effects of trastuzumab emtansine were fatigue, nausea, musculoskeletal pain, thrombocytopenia (low platelet counts), headache, increased liver enzyme levels, and constipation.[3]
Severe adverse events identified during the EMILIA trial included hepatotoxicity (liver damage), including rare cases of liver failure, hepatic encephalopathy, and nodular regenerative hyperplasia; heart damage (dysfunction of the left ventricle); interstitial lung disease, including acute interstitial pneumonitis; thrombocytopenia; and peripheral neuropathy.[3] Overall, trastuzumab emtansine was better tolerated than the control treatment, a combination of lapatinib (Tykerb) and capecitabine (Xeloda), with 43% of patients in the trastuzumab emtansine group experiencing severe toxic effects, versus 59% of those who received lapatinib/capecitabine; furthermore, fewer patients had to stop treatment due to adverse effects than with lapatinib or capecitabine.[3] Anemia, low platelet counts, and peripheral neuropathy were more common among patients who received trastuzumab emtansine, whereas heart damage and gastrointestinal effects, such as vomiting, diarrhea, and stomatitis, were more common with lapatinib/capecitabine.[3]
In the United States, Kadcyla carries black box warnings for liver toxicity, heart damage (reduction in left ventricular ejection fraction), and fetal harm if given to pregnant women.[3][11]
Mechanism of action
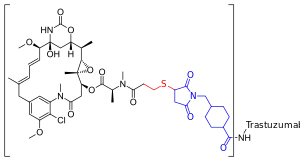
Trastuzumab emtansine is an antibody-drug conjugate (ADC), a combination between a monoclonal antibody and a small-molecule drug. Each molecule of trastuzumab emtansine consists of a single trastuzumab molecule with several molecules of DM1, a cytotoxic maytansinoid, attached.[12] SMCC, or succinimidyl trans-4-(maleimidylmethyl)cyclohexane-1-carboxylate, is a heterobifunctional crosslinker, a type of chemical reagent that contains two reactive functional groups, a succinimide ester and a maleimide. The succinimide group of SMCC reacts with the free amino group of a lysine residue in the trastuzumab molecule and the maleimide moiety of SMCC links to the free sulfhydryl group of DM1, forming a covalent bond between the antibody and the DM1. Each trastuzumab molecule may be linked to zero to eight DM1 molecules (3.5 on average).[12][13] DM1 binds at plus ends of cellular microtubules and thereby inhibits cell division in the target tumor cells.[14]
Trastuzumab alone stops growth of cancer cells by binding to the HER2 receptor, whereas trastuzumab emtansine undergoes receptor-mediated internalization into cells, is catabolized in lysosomes where DM1-containing catabolites are released and subsequently bind tubulin to cause mitotic arrest and cell death.[15] Trastuzumab binding to HER2 prevents homodimerization or heterodimerization (HER2/HER3) of the receptor, ultimately inhibiting the activation of MAPK and PI3K/AKT cellular signalling pathways. Because the monoclonal antibody targets HER2, and HER2 is only over-expressed in cancer cells, the conjugate delivers the cytotoxic agent DM1 specifically to tumor cells.[10] The conjugate is abbreviated "T-DM1".
Trastuzumab and DM1 work together, after cell entry via binding to HER2, by doing the following:[16]
- Inhibition of HER2 receptor signal
- Intervene in antibody-dependent cell-mediated cytotoxicity
- Prevent HER2 shedding of extracellular domain
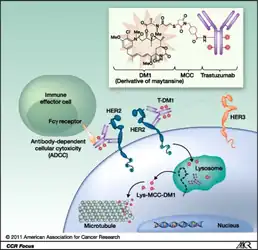 Structure of trastuzumab emtansine and mechanisms of action
Structure of trastuzumab emtansine and mechanisms of action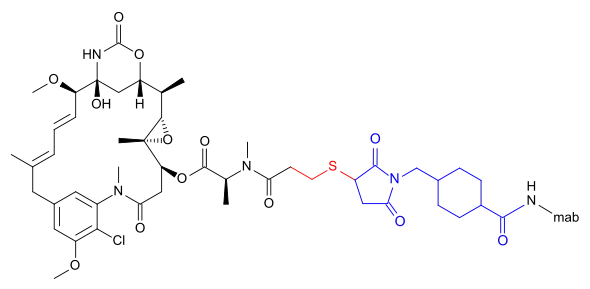 Schematic representation of trastuzumab emtansine. The maytansine skeleton is shown in black at left. The thioether group that makes mertansine is shown in red. The linker group that makes emtansine is shown in blue at right, bound to the amino group (HN–) of a lysine residue in the trastuzumab molecule (–mab).
Schematic representation of trastuzumab emtansine. The maytansine skeleton is shown in black at left. The thioether group that makes mertansine is shown in red. The linker group that makes emtansine is shown in blue at right, bound to the amino group (HN–) of a lysine residue in the trastuzumab molecule (–mab).
History
In 2013, ado-trastuzumab emtansine was approved in the United States for the treatment of adults with HER2-positive, metastatic breast cancer who previously received trastuzumab and a taxane, separately or in combination.[11][8]
Referred to as T-DM1 during clinical research, ado-trastuzumab emtansine was reviewed under the FDA's priority review program.[11]
The safety and effectiveness of ado-trastuzumab emtansine were evaluated in a clinical study of 991 patients randomly assigned to receive ado-trastuzumab emtansine or lapatinib plus capecitabine, another chemotherapy drug.[11] Patients received treatment until either the cancer progressed or the side effects became intolerable.[11] The study was designed to measure progression-free survival, the length of time patients lived without the cancer progressing, and overall survival, the length of time patients lived before death.[11]
Results showed that patients treated with ado-trastuzumab emtansine had a median progression-free survival of 9.6 months compared to 6.4 months in patients treated with lapatinib plus capecitabine.[11] The median overall survival was 30.9 months in the ado-trastuzumab emtansine group and 25.1 months in the lapatinib plus capecitabine group.[11]
The U.S. Food and Drug Administration (FDA) approved ado-trastuzumab emtansine in February 2013, and granted the application for Kadcyla to Genentech.[11]
In 2013, ado-trastuzumab emtansine was approved in the UK,[17] and the EU.[18]
In 2019, ado-trastuzumab emtansine was approved in the United States for the adjuvant treatment of patients with HER2-positive early breast cancer (EBC) who have residual invasive disease after neoadjuvant taxane and trastuzumab-based treatment.[19]
Approval was based on KATHERINE (NCT01772472[20]), a randomized, multicenter, open-label trial of 1486 patients with HER2-positive EBC.[19] Breast tumor samples were required to demonstrate HER2 overexpression defined as 3+ IHC or ISH amplification ratio ≥ 2.0 determined at a central laboratory using Ventana's PATHWAY anti-HER2-/neu (4B5) Rabbit Monoclonal Primary Antibody or INFORM HER2 Dual ISH DNA Probe Cocktail assays.[19] Patients were required to have had neoadjuvant taxane and trastuzumab-based therapy with residual invasive tumor in the breast and/or axillary lymph nodes.[19] Patients received radiotherapy and/or hormonal therapy concurrent with study treatment per local guidelines.[19] Patients were randomized (1:1) to receive ado-trastuzumab emtansine 3.6 mg/kg intravenously or trastuzumab 6 mg/kg intravenously on day 1 of a 21-day cycle for 14 cycles.[19]
The trial's primary endpoint was invasive disease-free survival (IDFS), defined as the time from the date of randomization to first occurrence of ipsilateral invasive breast tumor recurrence, ipsilateral local or regional invasive breast cancer recurrence, distant recurrence, contralateral invasive breast cancer, or death from any cause.[19] After a median follow-up of 40 months, the trial demonstrated a statistically significant improvement in IDFS in patients who received ado-trastuzumab emtansine compared with those who received trastuzumab (HR 0.50; 95% CI: 0.39, 0.64; p<0.0001).[19] Overall survival data were not mature at the time of the IDFS analysis.[19]
The U.S. Food and Drug Administration (FDA) granted the application for ado-trastuzumab emtansine priority review designation and breakthrough therapy designation for the adjuvant treatment of patients with HER2-positive early breast cancer who have residual disease after pre-operative systemic treatment.[19]
Society and culture
UK pricing
In the UK, Kadcyla was not recommended for use by the National Health Service by advisory body NICE, reportedly because an acceptable pricing agreement could not be reached with Roche.[21] Originally it cost £5,900 a month.[22] and NICE estimated it cost £166,000 per QALY[23] (well over the usual maximum). It has been funded by the English NHS Cancer Drugs Fund but in January 2015 it was proposed to remove it from the approved list.[24] After a secret discount was agreed by Roche the Cancer Drugs Fund will continue to fund it.[22]
In June 2017, the NHS Confederation and NHS Chief Executive Simon Stevens announced that the NHS would be offering Kadcyla to a limited number of women after striking a deal with Roche on the price.[25]
Name
In 2013, Kadcyla was approved in the United States with the generic name "ado-trastuzumab emtansine",[11][8] rather than the original United States Adopted Name (USAN) issued in 2009, "trastuzumab emtansine".[8] Trastuzumab is the anti-HER2 antibody; emtansine refers to the linker-drug (SMCC-DM1). The "ado-" prefix was added at the request of the FDA to help prevent dispensing errors.[26][8][27] During preclinical development and clinical trials, the drug was also known as trastuzumab-DM1 or trastuzumab-MCC-DM1 (after the codename for emtansine), both abbreviated T-DM1, and by the codename PRO132365.[1]
References
- 1 2 3 "NCI". www.cancer.gov. 2 February 2011. Archived from the original on 3 March 2021. Retrieved 13 January 2022.
- 1 2 3 4 5 "Kadcyla". Archived from the original on 29 April 2021. Retrieved 13 January 2022.
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 "Kadcyla- ado-trastuzumab emtansine injection, powder, lyophilized, for solution". DailyMed. 16 May 2019. Archived from the original on 4 August 2020. Retrieved 4 December 2019.
- ↑ Use During Pregnancy and Breastfeeding
- ↑ "Ado-trastuzumab emtansine (Kadcyla) Use During Pregnancy". Drugs.com. Archived from the original on 25 January 2021. Retrieved 13 January 2022.
- ↑ BNF 81: March-September 2021. BMJ Group and the Pharmaceutical Press. 2021. p. 931. ISBN 978-0857114105.
- ↑ "Kadcyla Prices, Coupons & Patient Assistance Programs". Drugs.com. Archived from the original on 9 February 2018. Retrieved 13 January 2022.
- 1 2 3 4 5 "Drug Approval Package: ado-trastuzumab emtansine". U.S. Food and Drug Administration (FDA). 22 February 2013. Archived from the original on 4 December 2019. Retrieved 3 December 2019.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - 1 2 Clinical trial number NCT00829166 for "A Study of Trastuzumab Emtansine Versus Capecitabine + Lapatinib in Participants With HER2-positive Locally Advanced or Metastatic Breast Cancer (EMILIA)" at ClinicalTrials.gov
- 1 2 Verma S, Miles D, Gianni L, et al. (November 2012). "Trastuzumab emtansine for HER2-positive advanced breast cancer". N. Engl. J. Med. 367 (19): 1783–91. doi:10.1056/NEJMoa1209124. PMC 5125250. PMID 23020162.
- 1 2 3 4 5 6 7 8 9 10 "FDA approves new treatment for late-stage breast cancer" (Press release). U.S. Food and Drug Administration (FDA). 22 February 2013. Archived from the original on 12 January 2017. Retrieved 22 February 2013.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - 1 2 Girish S, Gupta M, Wang B, et al. (May 2012). "Clinical pharmacology of trastuzumab emtansine (T-DM1): an antibody-drug conjugate in development for the treatment of HER2-positive cancer". Cancer Chemother. Pharmacol. 69 (5): 1229–40. doi:10.1007/s00280-011-1817-3. PMC 3337408. PMID 22271209.
- ↑ Lewis Phillips GD, Li G, Dugger DL, et al. (November 2008). "Targeting HER2-positive breast cancer with trastuzumab-DM1, an antibody-cytotoxic drug conjugate". Cancer Res. 68 (22): 9280–90. doi:10.1158/0008-5472.CAN-08-1776. PMID 19010901.
- ↑ Lopus M (August 2011). "Antibody-DM1 conjugates as cancer therapeutics". Cancer Letters. 307 (2): 113–118. doi:10.1016/j.canlet.2011.03.017. PMC 3105156. PMID 21481526.
- ↑ Teicher BA, Doroshow JH (November 2012). "The promise of antibody-drug conjugates". N. Engl. J. Med. 367 (19): 1847–8. doi:10.1056/NEJMe1211736. PMID 23134386.
- ↑ Barok, Mark; Joensuu, Heikki; Isola, Jorma (2014). "Trastuzumab emtansine: mechanisms of action and drug resistance". Breast Cancer Research : BCR. 16 (2): 209. doi:10.1186/bcr3621. ISSN 1465-5411. Archived from the original on 11 January 2022. Retrieved 28 December 2021.
- ↑ "Kadcyla 100 mg Powder for Concentrate for Solution for Infusion - Summary of Product Characteristics (SmPC)". electronic medicines compendium (emc). 19 November 2019. Archived from the original on 4 December 2019. Retrieved 3 December 2019.
- ↑ "Kadcyla EPAR". European Medicines Agency (EMA). 17 September 2018. Archived from the original on 29 April 2021. Retrieved 24 September 2021.
- 1 2 3 4 5 6 7 8 9 10 "FDA approves ado-trastuzumab emtansine for early breast cancer". U.S. Food and Drug Administration (FDA) (Press release). 6 May 2019. Archived from the original on 28 September 2019. Retrieved 3 December 2019.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - ↑ Clinical trial number NCT01772472 for "A Study of Trastuzumab Emtansine Versus Trastuzumab as Adjuvant Therapy in Patients With HER2-Positive Breast Cancer Who Have Residual Tumor in the Breast or Axillary Lymph Nodes Following Preoperative Therapy (KATHERINE)" at ClinicalTrials.gov
- ↑ Triggle N (8 August 2014). "NHS says no to new breast cancer drug Kadcyla". BBC News Online. Archived from the original on 12 March 2021. Retrieved 8 August 2014.
- 1 2 "Breast cancer drug Kadcyla to remain on NHS after manufacturer lowers price. Nov 2015". Archived from the original on 8 November 2020. Retrieved 24 September 2021.
- ↑ "Pressure grows on Roche to lower breast cancer drug price. Aug 2014". Archived from the original on 9 June 2021. Retrieved 24 September 2021.
- ↑ "David Cameron's flagship Cancer Drugs Fund 'is a waste of NHS cash'". Guardian. 10 January 2015. Archived from the original on 15 January 2015. Retrieved 11 January 2015.
- ↑ "NHS U-turn sees breast cancer drug Kadcyla approved for use". NursingNotes. 16 June 2017. Archived from the original on 3 June 2019. Retrieved 16 June 2017.
- ↑ "Drug Safety Communication: FDA warns about potential medication errors resulting from confusion regarding nonproprietary name for breast cancer drug Kadcyla (ado-trastuzumab emtansine)". U.S. Food and Drug Administration (FDA). 16 January 2016. Archived from the original on 4 December 2019. Retrieved 3 December 2019.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - ↑ Kim TE, Pazdur R (2013). Summary Review for Regulatory Action (PDF) (Technical report). U.S. Food and Drug Administration. p. 8. Retrieved 22 February 2013.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
External links
| Identifiers: |
|---|
- "Trastuzumab emtansine". Drug Information Portal. U.S. National Library of Medicine. Archived from the original on 28 January 2021. Retrieved 24 September 2021.