Adipose tissue macrophages
Adipose tissue macrophages (abbr. ATMs) comprise tissue resident macrophages present in adipose tissue. Adipose tissue apart from adipocytes is composed of the stromal vascular fraction (SVF) of cells including preadipocytes, fibroblasts, vascular endothelial cells and variety of immune cells. The latter ones are composed of mast cells, eosinophils, B cells, T cells and macrophages.[1] The number of macrophages within adipose tissue differs depending on the metabolic status. As discovered by Rudolph Leibel and Anthony Ferrante et al. in 2003 at Columbia University, the percentage of macrophages within adipose tissue ranges from 10% in lean mice and humans up to 50% in extremely obese, leptin deficient mice and almost 40% in obese humans.[2] Increased number of adipose tissue macrophages correlates with increased adipose tissue production of proinflammatory molecules and might therefore contribute to the pathophysiological consequences of obesity (e.g. insulin resistance, type 2 diabetes).[3]
M1/M2 macrophage polarization
Macrophages are remarkably plastic cells which in order to adapt to different tissue microenvironments can assume a range of different phenotypes. Accordingly, macrophages can exhibit either pro- or anti-inflammatory phenotypes and are routinely classified into M1 (classically activated) phenotype and M2 (alternatively activated) phenotype.[4] According to this classification, macrophages acquire M1 phenotype following in vitro stimulation with interferon gamma (IFN-γ) alone or in combination with TLR ligands (e.g. lipopolysaccharide (LPS)) whereas macrophages acquire M2 phenotype after in vitro exposure to IL-4 and IL-13. M1 macrophages secrete high levels of proinflammatory cytokines (e.g. tumor necrosis factor (TNF-α), IL-6, IL-1β) and generate reactive oxygen and nitrogen species such as nitric oxide via activation of inducible nitric oxide synthase (iNOS). Conversely, M2 macrophages activate arginase 1 (Arg1) that blocks iNOS activity and therefore inhibits nitric oxide production. They also secrete anti-inflammatory cytokines (e.g. IL-10, TGF-β, IL-4) essential for inflammatory response resolution. M1 macrophages are microbicidal and tumoricidal, and stimulate adaptive immune response. M2 macrophages are associated with anti-inflammatory and homeostatic functions linked to wound healing. However, in this classification system, M1 and M2 macrophages are regarded as two extreme phenotypes. For example, macrophages stimulated with IL-4 and IL-13 are defined as M2a, whereas macrophages stimulated with LPS and apoptotic cells as M2b and macrophages stimulated with IL-10, transforming growth factor-β (TGF-β) or glucocorticoids as M2c.[5] In adipose tissue, distinction between M1 and M2 macrophage polarization can be monitored by assessing the expression of selected markers. Macrophages displaying M1 phenotype have been characterized by expression of F4/80, CD11c and iNOS whereas macrophages displaying M2 phenotype have been characterized by expression of F4/80, CD301 and Arg1.[6] Adiopose tissue macrophage polarization was summarized in a recent review article Appari M et al., et al.[7]
Adipose tissue macrophages and obesity
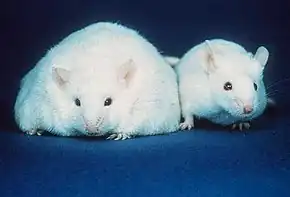
Increased recruitment of macrophages into adipose tissue is multifactoral.[8] Adipocyte cell death observed within pathologically expanding adipose tissue is one of the factors. Macrophages are specialized phagocytes that remove dying or dead cells or cellular debris. Within adipose tissue, presence of dead adipocytes is a hallmark of obesity. Macrophages surrounding dying or dead adipocytes form crown-like structures (CLSs), identified by the absence of perilipin staining.[9]
In addition to increased number of macrophages within adipose tissue, obesity also induces a phenotypic switch in these cells toward the classically activated (M1) phenotype.[10] Moreover, expression of inflammatory cytokines such as TNF-α is mostly derived from macrophages rather than adipocytes.[11] It has been proposed that their presence contributes to the development of insulin resistance (IR) and diabetes type-2. Early and late stages of diet-induced obesity can also induce macrophage populations that are not representative of M1 or M2 phenotypes, including metabolically activate macrophages (MMe) and oxidized macrophages (Mox), both associated with IR. [12]
Adipose tissue macrophages isolated from obese patients express growth factors, cytokines, chemokines, and proteolytic enzymes involved in the regulation of tumor growth, angiogenesis, invasion, and metastatic spread, and resemble macrophages present in tumor stroma.[13]
Adipose tissue macrophages and weight loss
Acute weight loss is also associated with increased, yet transient recruitment of macrophages into adipose tissue. However the recruited macrophages do not promote inflammatory response but rather regulate lipolysis. Recruited macrophages are characterized by higher expression of scavenger receptors (i.e. CD36 and macrophage scavenger receptor 1 (MSR1)) and lipid-handling genes (i.e. adipose differentiation-related protein (Adfp), fatty acid-binding protein 4 (Fabp4), ApoE and ABCA1), and increased accumulation of Oil Red O-positive lipids. In this case, release of free fatty acids (FFAs) serves as a signal for macrophage recruitment.[14][15]
It has been shown in mice that adipose tissue macrophages regulate the age-related reduction of adipocyte lipolysis during ageing by lowering the bioavailability of noradrenaline. Inhibition of MAOA, an enzyme known to degrade noradrenaline, reversed the reduction in noradrenaline concentration and restored lipolysis in mice.[16]
Adipose tissue macrophages and tumor growth
Macrophages within tumor stroma, so called tumor-associated macrophages (TAMs) promote tumor growth and metastasis.[17] Tumor-associated macrophage infiltration correlates with poor prognosis in patients with breast, cervix, bladder and brain cancers.[18] Pathophysiological interaction between tumor-associated macrophages and surrounding cells, such as endothelial cells promote tumor progression. In 1971, Judah Folkman proposed that angiogenesis plays essential role in tumor growth.[19] Macrophages secrete many pro-angiogenic factors including vascular endothelial growth factor (VEGF), TNF-α, granulocyte macrophage colony-stimulating factor (GM-CSF) and IL-1 and IL-6.[20] Additionally it has been shown that adipose tissue surrounding certain tumors or metastases to the lymph nodes, which are embedded in adipose tissue, fuels tumor growth by serving as a depot for adipose tissue macrophages that stimulate angiogenesis and resemble TAMs.[21][22][23][24]
References
- ↑ Schipper, HS; Prakken, B; Kalkhoven, E; Boes, M (2012). "Adipose tissue-resident immune cells: key players in immunometabolism". Trends Endocrinol Metab. 23 (8): 407–15. doi:10.1016/j.tem.2012.05.011. PMID 22795937. S2CID 38312588.
- ↑ Weisberg, SP; McCann, D; Desai, M; Rosenbaum, M; Leibel, RL; Ferrante, AW (2003). "Obesity is associated with macrophage accumulation in adipose tissue". Journal of Clinical Investigation. 112 (12): 1796–808. doi:10.1172/jci200319246. PMC 296995. PMID 14679176.
- ↑ Lumeng, CN; Bodzin, JL; Saltiel, AR (2007). "Obesity induces a phenotypic switch in adipose tissue macrophage polarization". J Clin Invest. 117 (1): 175–84. doi:10.1172/jci29881. PMC 1716210. PMID 17200717.
- ↑ Mosser, DM; Edwards, JP (2008). "Exploring the full spectrum of macrophage activation". Nat Rev Immunol. 8 (12): 958–69. doi:10.1038/nri2448. PMC 2724991. PMID 19029990.
- ↑ Mantovani, A; Sica, A; Sozzani, S; Allavena, P; Vecchi, A; Locati, M (2004). "The chemokine system in diverse forms of macrophage activation and polarization". Trends Immunol. 25 (12): 677–86. doi:10.1016/j.it.2004.09.015. PMID 15530839.
- ↑ Eagle, AR; Chawla, A (2010). "In obesity and weight loss, all roads lead to the mighty macrophage". Journal of Clinical Investigation. 120 (10): 3437–40. doi:10.1172/jci44721. PMC 2947244. PMID 20877005.
- ↑ Appari, M; Channon, KM; McNeill, E (2017). "Metabolic Regulation of Adipose Tissue Macrophage Function in Obesity and Diabetes". Antioxid Redox Signal. 29 (3): 297–312. doi:10.1089/ars.2017.7060. PMC 6012981. PMID 28661198.
- ↑ Surmi, BK; Hasty, AH (2008). "Macrophage infiltration into adipose tissue: initiation, propagation and remodeling". Future Lipidol. 3 (5): 545–56. doi:10.2217/17460875.3.5.545. PMC 2575346. PMID 18978945.
- ↑ Cinti, S; Mitchell, G; Barbatelli, G; Murano, I; Ceresi, E; Faloia, E; et al. (2005). "Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans". J Lipid Res. 46 (11): 2347–55. doi:10.1194/jlr.m500294-jlr200. PMID 16150820.
- ↑ Lumeng, CN; Bodzin, JL; Saltiel, AR (2007). "Obesity induces a phenotypic switch in adipose tissue macrophage polarization". J Clin Invest. 117 (1): 175–84. doi:10.1172/jci29881. PMC 1716210. PMID 17200717.
- ↑ Weisberg, SP; McCann, D; Desai, M; Rosenbaum, M; Leibel, RL; Ferrante, AW (2003). "Obesity is associated with macrophage accumulation in adipose tissue". Journal of Clinical Investigation. 112 (12): 1796–808. doi:10.1172/jci200319246. PMC 296995. PMID 14679176.
- ↑ Skuratovskaia, D; Vulf, M; Khaziakhmatova, O (2020). "Tissue-Specific Role of Macrophages in Noninfectious Inflammatory Disorders". Biomedicines. 8 (10): 400. doi:10.3390/biomedicines8100400. PMC 7600904. PMID 33050138.
- ↑ Mayi, TH; Daoudi, M; Derudas, B; Gross, B; Bories, G; Wouters, K; et al. (2012). "Human adipose tissue macrophages display activation of cancer-related pathways". J Biol Chem. 287 (26): 21904–13. doi:10.1074/jbc.m111.315200. PMC 3381151. PMID 22511784.
- ↑ Eagle, AR; Chawla, A (2010). "In obesity and weight loss, all roads lead to the mighty macrophage". Journal of Clinical Investigation. 120 (10): 3437–40. doi:10.1172/jci44721. PMC 2947244. PMID 20877005.
- ↑ Kosteli, A; Sugaru, E; Haemmerle, G; Martin, JF; Lei, J; Zechner, R; et al. (2010). "Weight loss and lipolysis promote a dynamic immune response in murine adipose tissue". Journal of Clinical Investigation. 120 (10): 3466–79. doi:10.1172/jci42845. PMC 2947229. PMID 20877011.
- ↑ Camell, Christina D.; Sander, Jil; Spadaro, Olga; Lee, Aileen; Nguyen, Kim Y.; Wing, Allison; Goldberg, Emily L.; Youm, Yun-Hee; Brown, Chester W.; Elsworth, John; Rodeheffer, Matthew S.; Schultze, Joachim L.; Deep Dixit, Vishwa (2017). "Inflammasome-driven catecholamine catabolism in macrophages blunts lipolysis during ageing". Nature. 550 (7674): 119–123. Bibcode:2017Natur.550..119C. doi:10.1038/nature24022. ISSN 0028-0836. PMC 5718149. PMID 28953873.
- ↑ Biswas, SK; Sica, A; Lewis, CE (2008). "Plasticity of macrophage function during tumor progression: regulation by distinct molecular mechanisms". J Immunol. 180 (4): 2011–7. doi:10.4049/jimmunol.180.4.2011. PMID 18250403.
- ↑ Bingle, L; Brown, NJ; Lewis, CE (2002). "The role of tumour-associated macrophages in tumour progression: implications for new anticancer therapies". J Pathol. 196 (3): 254–65. doi:10.1002/path.1027. PMID 11857487. S2CID 8481685.
- ↑ Cao, YH; Langer, R (2008). "A review of Judah Folkman's remarkable achievements in biomedicine". Proc Natl Acad Sci USA. 105 (36): 13203–5. Bibcode:2008PNAS..10513203C. doi:10.1073/pnas.0806582105. PMC 2533169. PMID 18772371.
- ↑ Lin, EY; Li, JF; Gnatovskiy, L; Deng, Y; Zhu, L; Grzesik, DA; et al. (2006). "Macrophages regulate the angiogenic switch in a mouse model of breast cancer". Cancer Res. 66 (23): 11238–46. doi:10.1158/0008-5472.can-06-1278. PMID 17114237.
- ↑ Wagner, M; Bjerkvig, R; Wiig, H; Melero-Martin, JM; Lin, RZ; Klagsbrun, M; et al. (2012). "Inflamed tumor-associated adipose tissue is a depot for macrophages that stimulate tumor growth and angiogenesis". Angiogenesis. 15 (3): 481–95. doi:10.1007/s10456-012-9276-y. PMC 3619408. PMID 22614697.
- ↑ Wagner, M; Bjerkvig, R; Wiig, H; Dudley, AC (2013). "Loss of adipocyte specification and necrosis augment tumor-associated inflammation". Adipocyte. 2 (3): 176–83. doi:10.4161/adip.24472. PMC 3756107. PMID 23991365.
- ↑ Wagner, M; Dudley, AC (2013). "A three-party alliance in solid tumors: Adipocytes, macrophages and vascular endothelial cells". Adipocyte. 2 (2): 67–73. doi:10.4161/adip.23016. PMC 3661111. PMID 23805401.
- ↑ Wagner, Marek; Steinskog, Eli Sihn; Wiig, Helge (October 2019). "Blockade of Lymphangiogenesis Shapes Tumor-Promoting Adipose Tissue Inflammation". The American Journal of Pathology. 189 (10): 2102–2114. doi:10.1016/j.ajpath.2019.06.010. PMID 31369756.
- Appari, M; Channon, KM; McNeill, E (2017). "Metabolic Regulation of Adipose Tissue Macrophage Function in Obesity and Diabetes". Antioxid Redox Signal. 29 (3): 297–312. doi:10.1089/ars.2017.7060. PMC 6012981. PMID 28661198.