Bacteriochlorophyll
 | |
| Names | |
|---|---|
| IUPAC name
[methyl (3S,4S,13R,14R,21R)-9-acetyl-14-ethyl-4,8,13,18-tetramethyl-20-oxo-3-(3-oxo-3-([(2E,7R,11R)-3,7,11,15-tetramethylhexadec-2-en-1-yl]oxy)propyl)-13,14-dihydrophorbine-21-carboxylatato(2−)-kappa4N23,N24,N25,N26]magnesium | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| KEGG | |
PubChem CID |
|
InChI
| |
SMILES
| |
| Properties | |
Chemical formula |
C55H74MgN4O6 |
| Molar mass | 911.504 g/mol |
| Appearance | Light green to blue-green powder |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Bacteriochlorophylls (BChl) are photosynthetic pigments that occur in various phototrophic bacteria. They were discovered by C. B. van Niel in 1932.[1] They are related to chlorophylls, which are the primary pigments in plants, algae, and cyanobacteria. Organisms that contain bacteriochlorophyll conduct photosynthesis to sustain their energy requirements, but do not produce oxygen as a byproduct. They use wavelengths of light not absorbed by plants or Cyanobacteria. Replacement of Mg2+
with protons gives bacteriophaeophytin (BPh), the phaeophytin form.
| Pigment | Bacterial group | in vivo infrared absorption maximum (nm) |
|---|---|---|
| Bacteriochlorophyll a | Purple bacteria, Heliobacteria, Green Sulfur Bacteria, Chloroflexi, Chloracidobacterium thermophilum[2] | 805, 830–890 |
| Bacteriochlorophyll b | Purple bacteria | 835–850, 1020–1040 |
| Bacteriochlorophyll c | Green sulfur bacteria, Chloroflexi, C. thermophilum | 745–755 |
| Bacteriochlorophyll cs | Chloroflexi | 740 |
| Bacteriochlorophyll d | Green sulfur bacteria | 705–740 |
| Bacteriochlorophyll e | Green sulfur bacteria | 719–726 |
| Bacteriochlorophyll f | Green sulfur bacteria (currently found only through mutation; natural may exist)[3] | 700–710 |
| Bacteriochlorophyll g | Heliobacteria | 670, 788 |
- Structures of some bacteriochlorophylls
 bacteriochlorophyll a
bacteriochlorophyll a bacteriochlorophyll b
bacteriochlorophyll b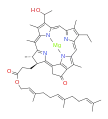 bacteriochlorophyll c
bacteriochlorophyll c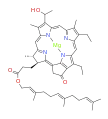 bacteriochlorophyll d
bacteriochlorophyll d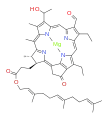 bacteriochlorophyll e
bacteriochlorophyll e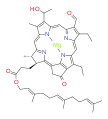 bacteriochlorophyll f
bacteriochlorophyll f bacteriochlorophyll g
bacteriochlorophyll g
Structure
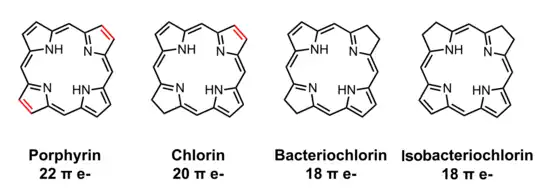
Bacteriochlorophylls a, b, and g are bacteriochlorins, meaning their molecules have a bacteriochlorin macrocycle ring with two reduced pyrrole rings (B and D). Bacteriochlorophylls c, d, e, and f are chlorins, meaning their molecules have a chlorin macrocycle ring with one reduced pyrrole ring (D).[4]
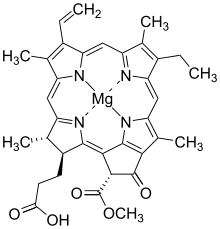
Bacteriochlorophylls c to f occur in the form of closely related homologs with different alkyl groups attached to pyrrole rings B and C and are illustrated above in their simplest versions, esterified with the sesquiterpene alcohol farnesol.[5] Bacteriochlorophyll g has a vinyl group in ring (A).[6]
Biosynthesis
There are a large number of known bacteriochlorophylls[4][7] but all have features in common since the biosynthetic pathway involves chlorophyllide a as an intermediate.[8] Isobacteriochlorins, in contrast, are biosynthesised from uroporphyrinogen III in a separate pathway that leads, for example, to siroheme, cofactor F430 and cobalamin.
References
- ↑ Niel, C. B. (1932). "On the morphology and physiology of the purple and green sulphur bacteria". Archiv für Mikrobiologie. 3: 1–112. doi:10.1007/BF00454965. S2CID 19597530.
- ↑ Bryant DA, et al. (2007-07-27), "Candidatus Chloracidobacterium thermophilum: An Aerobic Phototrophic Acidobacterium" (PDF), Science, 317 (5837): 523–526, Bibcode:2007Sci...317..523B, doi:10.1126/science.1143236, PMID 17656724, S2CID 20419870
- ↑ Vogl K, et al. (2012-08-10). "Bacteriochlorophyll f: properties of chlorosomes containing the "forbidden chlorophyll"". Front. Microbiol. 3: article 298, pages 1–12. doi:10.3389/fmicb.2012.00298. PMC 3415949. PMID 22908012.
- 1 2 Senge, Mathias O.; Smith, Kevin M. (2004). "Biosynthesis and Structures of the Bacteriochlorophylls". Anoxygenic Photosynthetic Bacteria. Advances in Photosynthesis and Respiration. Vol. 2. pp. 137–151. doi:10.1007/0-306-47954-0_8. ISBN 0-7923-3681-X.
- ↑ Harada, Jiro; Shibata, Yutaka; Teramura, Misato; Mizoguchi, Tadashi; Kinoshita, Yusuke; Yamamoto, Ken; Tamiaki, Hitoshi (2018). "In Vivo Energy Transfer from Bacteriochlorophyll c , d , e , or f to Bacteriochlorophyll a in Wild-Type and Mutant Cells of the Green Sulfur Bacterium Chlorobaculum limnaeum". ChemPhotoChem. 2 (3): 190–195. doi:10.1002/cptc.201700164.
- ↑ Tsukatani, Yusuke; Yamamoto, Haruki; Mizoguchi, Tadashi; Fujita, Yuichi; Tamiaki, Hitoshi (2013). "Completion of biosynthetic pathways for bacteriochlorophyll g in Heliobacterium modesticaldum: The C8-ethylidene group formation". Biochimica et Biophysica Acta (BBA) - Bioenergetics. 1827 (10): 1200–1204. doi:10.1016/j.bbabio.2013.06.007. PMID 23820336.
- ↑ Chew, Aline Gomez Maqueo; Bryant, Donald A. (2007). "Chlorophyll Biosynthesis in Bacteria: The Origins of Structural and Functional Diversity". Annual Review of Microbiology. 61: 113–129. doi:10.1146/annurev.micro.61.080706.093242. PMID 17506685.
- ↑ Willows, Robert D. (2003). "Biosynthesis of chlorophylls from protoporphyrin IX". Natural Product Reports. 20 (6): 327–341. doi:10.1039/B110549N. PMID 12828371.