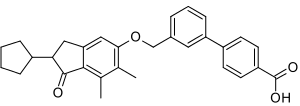
Biphenylindanone A
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C30H30O4 |
| Molar mass | 454.566 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (verify) | |
Biphenylindanone A (BINA, LS-193,571) is a research agent which acts as a potent and selective positive allosteric modulator for the group II metabotropic glutamate receptor subtype mGluR2.
In animal studies it showed anxiolytic and antipsychotic effects,[1] and blocked the effects produced by the hallucinogenic drug DOB. BINA and other selective mGluR2 positive modulators have therefore been suggested as a novel class of drugs for the treatment of schizophrenia which may have superior properties to traditional antipsychotic drugs.[2]
BINA decreases cocaine self-administration in rats, with no effect on food self-administration, and is in regard to this discrimination superior to the mGluR2/3 agonist LY-379,268.[3]
References
- ↑ Galici R, Jones CK, Hemstapat K, Nong Y, Echemendia NG, Williams LC, et al. (July 2006). "Biphenyl-indanone A, a positive allosteric modulator of the metabotropic glutamate receptor subtype 2, has antipsychotic- and anxiolytic-like effects in mice". The Journal of Pharmacology and Experimental Therapeutics. 318 (1): 173–85. doi:10.1124/jpet.106.102046. PMID 16608916. S2CID 14653620.
- ↑ Benneyworth MA, Xiang Z, Smith RL, Garcia EE, Conn PJ, Sanders-Bush E (August 2007). "A selective positive allosteric modulator of metabotropic glutamate receptor subtype 2 blocks a hallucinogenic drug model of psychosis". Molecular Pharmacology. 72 (2): 477–84. doi:10.1124/mol.107.035170. PMID 17526600. S2CID 3097502.
- ↑ Jin X, Semenova S, Yang L, Ardecky R, Sheffler DJ, Dahl R, et al. (September 2010). "The mGluR2 positive allosteric modulator BINA decreases cocaine self-administration and cue-induced cocaine-seeking and counteracts cocaine-induced enhancement of brain reward function in rats". Neuropsychopharmacology. 35 (10): 2021–36. doi:10.1038/npp.2010.82. PMC 2922422. PMID 20555310.
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.