Decoglurant
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| Chemical and physical data | |
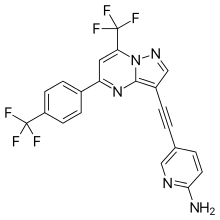
| Formula | C21H11F6N5 |
| Molar mass | 447.344 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Decoglurant (INN) (code name RG1578, RO4995819) is a negative allosteric modulator of the mGlu2 and mGlu3 receptors which was under development by Roche for the adjunctive treatment of major depressive disorder.[1][2] Decoglurant progressed as far as phase II clinical trials[1][2] but was ultimately discontinued from further development due to disappointing efficacy results.[3][4]
See also
References
- 1 2 "Roche – Pipeline". 2014. Retrieved 2014-08-01.
- 1 2 "Roche Group Development Pipeline" (PDF). 2014. Archived from the original (PDF) on 2014-08-08. Retrieved 2014-08-01.
- ↑ "Roche – Pipeline" (PDF). 2015. Archived from the original (PDF) on 2015-05-01. Retrieved 2015-05-14.
- ↑ Lawrence J (March 2015). "The Secret Life of ketamine". The Pharmaceutical Journal. 294 (7854/5).
External links
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.