Brincidofovir
 | |
| Clinical data | |
|---|---|
| Trade names | Tembexa |
| Other names | CMX001; Cidofovir-HDP; hexadecyloxypropyl-cidofovir |
| License data | |
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
IUPAC name
| |
| CAS Number |
|
| PubChem CID | |
| DrugBank |
|
| ChemSpider | |
| UNII |
|
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
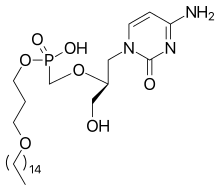
| Formula | C27H52N3O7P |
| Molar mass | 561.701 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Brincidofovir, sold under the brand name Tembexa, is an antiviral drug used to treat smallpox. Brincidofovir is a prodrug of cidofovir.[2] Conjugated to a lipid, the compound is designed to release cidofovir intracellularly, allowing for higher intracellular and lower plasma concentrations of cidofovir, effectively increasing its activity against dsDNA viruses, as well as oral bioavailability.[3]
The most common side effects include diarrhea, nausea, vomiting, and abdominal pain.[1]
Brincidofovir was approved for medical use in the United States in June 2021.[1]
Medical uses
Brincidofovir is indicated for the treatment of human smallpox disease caused by variola virus.[1][4]
History
Because smallpox is eradicated, the effectiveness of brincidofovir was studied in animals infected with viruses that are closely related to the variola virus.[1] Effectiveness was determined by measuring animals' survival at the end of the studies.[1]
Safety information to support approval of brincidofovir was derived from clinical trials of the drug for a non-smallpox indication, primarily from patients who received hematopoietic stem cell transplants.[1]
The U.S. Food and Drug Administration (FDA) granted the application for brincidofovir priority review, fast track, and orphan drug designations.[1] The FDA approved brincidofovir under the agency's Animal Rule, which allows findings from adequate and well-controlled animal efficacy studies to serve as the basis of an approval when it is not feasible or ethical to conduct efficacy trials in humans.[1]
Ethical considerations
Brincidofovir (CMX001) was the subject of widespread social media campaigning in 2014, which was then picked up by national news sources about a boy with an adenovirus infection following a bone marrow transplant.[5] The family requested legal access to the still-unapproved drug outside of any clinical trial, and Chimerix initially denied the request. After a short and intense media campaign, Chimerix got permission from the FDA to start a limited open-label trial which allowed the boy to receive the drug.[5] This media event sparked a debate on the ethics of using social media, the allocation of limited resources of a small company, and the emphasis on the individual over the group. The new use of any drug has the potential to interfere with the process to get the drug approved and widely marketed, through means such as consuming limited staff time that may be needed elsewhere – staff time that has the potential to save thousands of lives in the long-term, rather than one life now – overwhelming manufacturing capabilities, or by causing adverse effects or even death.[5] These adverse events are more likely during these programs, because the people seeking access are usually much sicker than most, and problems experienced by these people can result in an unfavorable and inaccurate perception of the drug's safety profile.[5] In this case, the boy recovered from the infection in 2014, and died in 2016 from complications of cancer.[6]
Brincidofovir is one of several experimental drugs administered to a small number of patients to treat Ebola virus disease during the 2014 outbreak. The WHO published a report on the ethics of using unregistered interventions to treat Ebola, where they concluded that "In the particular context of the current Ebola outbreak in West Africa, it is ethically acceptable to offer unproven interventions that have shown promising results in the laboratory and in animal models but have not yet been evaluated for safety and efficacy in humans as potential treatment or prevention."[7]
Research
Brincidofovir is under investigation for the treatment of cytomegalovirus, adenovirus, poxvirus, and ebolavirus infections.[8]
Adenovirus and cytomegalovirus
As of 2014, brincidofovir is in Phase III clinical trials for use in humans against cytomegalovirus and adenovirus. Preliminary safety data from a database of 1000 patients supported progression into later phase trials,[9] Chimerix announced in December 2015 that the Phase III trials for use of the drug in preventing cytomegalovirus infection in stem cell transplant patients had failed, and in February 2016 shut down two other late-stage trials for use of the drug in preventing infection after kidney transplants. Brincidofovir is not yet FDA approved for adenovirus or cytomegalovirus due to lack of efficacy in clinical trials.[10] In a trial of brincidofovir for patients with CMV brincidofovir was associated with a 15.5% week 24 all-cause mortality compared with 10.1% among placebo recipients. Additionally brincidofovir was associated with increased serious adverse events (57.1% versus 37.6%) compared with placebo.[10] Brincidofovir was initially offered via an FDA expanded access trial; however as of May 9, 2019, Chimerix discontinued clinical trials of brincidofovir for the treatment of adenovirus and discontinued the expanded access program in 2019.[11]
Ebola
After initial studies by the Centers for Disease Control and Prevention (CDC, Atlanta, GA, USA) in cell culture models,[12] on October 6, 2014, Chimerix received an FDA authorization for emergency investigational new drug applications of brincidofovir for the treatment of Ebola virus disease. Brincidofovir was administered to the first patient diagnosed in the Ebola virus disease outbreak in the US in 2014.[13][14] The patient was given the drug starting six days after hospital admission when he was already critically ill; he died four days later.[15][16] Brincidofovir was also given to Ebola patient Ashoka Mukpo at the Nebraska Medical Center, who had developed the disease and then was pronounced Ebola-free and released from the Center on 22 October 2014.[17]
In October 2014, Chimerix reported it had been given approval by the FDA to start Phase 2 trials in patients infected with ebolaviruses for brincidofovir's safety, tolerability, and efficacy.[18] Organised by a team of scientists at the University of Oxford, including Peter Horby, Jake Dunning, Laura Merson and Trudie Lang,[19] a trial commenced during January 2015 in Liberia,[20] but was subsequently discontinued. Because of a lack of suitable subjects in Liberia, Oxford University and Médecins Sans Frontières planned to extend the trial to Sierra Leone, where there were still Ebola cases; but on the 30th of January 2015, the manufacturer decided to withdraw support for the trial and end discussion of future trials.[21][22]
Animals
In animal trials brincidofovir has shown activity against cytomegalovirus, adenoviruses, BK virus, poxviruses, and herpes simplex viruses.[2][23] Brincidofovir appears to have potential for the treatment of Ebola virus disease, which is somewhat paradoxical, as ebolaviruses are RNA viruses and thus do not contain DNA as the above mentioned viruses. [12][24]
References
- 1 2 3 4 5 6 7 8 9 "FDA approves drug to treat smallpox". U.S. Food and Drug Administration (FDA). 4 June 2021. Retrieved 7 June 2021.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - 1 2 "Brincidofovir (CMX001)". Chimerix. Archived from the original on 20 March 2014.
- ↑ Florescu DF, Keck MA (October 2014). "Development of CMX001 (Brincidofovir) for the treatment of serious diseases or conditions caused by dsDNA viruses". Expert Review of Anti-Infective Therapy. 12 (10): 1171–1178. doi:10.1586/14787210.2014.948847. PMID 25120093. S2CID 25854860.
- ↑ Birnkrant D (4 June 2021). "Brincidofovir: NDA Approval – Animal Efficacy" (PDF). Center for Drug Evaluation and Research. U.S. Food and Drug Administration.
- 1 2 3 4 Darrow JJ, Sarpatwari A, Avorn J, Kesselheim AS (January 2015). "Practical, legal, and ethical issues in expanded access to investigational drugs". The New England Journal of Medicine. 372 (3): 279–286. doi:10.1056/nejmhle1409465. PMID 25587952.
- ↑ "Josh Hardy, Va. Boy Who Inspired Social Media Campaign, Dies". NBC4 Washington. 22 October 2016. Retrieved 21 May 2018.
- ↑ Report of an advisory panel to WHO. "Ethical considerations for use of unregistered interventions for Ebola virus disease". Archived from the original on 23 August 2014. Retrieved 8 October 2014.
- ↑ Lanier R, Trost L, Tippin T, Lampert B, Robertson A, Foster S, et al. (December 2010). "Development of CMX001 for the Treatment of Poxvirus Infections". Viruses. 2 (12): 2740–2762. doi:10.3390/v2122740. PMC 3077800. PMID 21499452.
- ↑ "Brincidofovor for Ebola". Chimerix. Archived from the original on 9 October 2014.
- 1 2 Marty FM, Winston DJ, Chemaly RF, Mullane KM, Shore TB, Papanicolaou GA, et al. (SUPPRESS Trial Clinical Study Group) (February 2019). "A Randomized, Double-Blind, Placebo-Controlled Phase 3 Trial of Oral Brincidofovir for Cytomegalovirus Prophylaxis in Allogeneic Hematopoietic Cell Transplantation". Biology of Blood and Marrow Transplantation. 25 (2): 369–381. doi:10.1016/j.bbmt.2018.09.038. PMC 8196624. PMID 30292744.
- ↑ "Chimerix Expanded Access". Chimerix, Inc.
- 1 2 McMullan LK, Flint M, Dyall J, Albariño C, Olinger GG, Foster S, et al. (January 2016). "The lipid moiety of brincidofovir is required for in vitro antiviral activity against Ebola virus". Antiviral Research. 125: 71–78. doi:10.1016/j.antiviral.2015.10.010. PMID 26526586.
- ↑ "Chimerix Announces Emergency Investigational New Drug Applications for Brincidofovir Authorized by FDA for Patients With Ebola Virus Disease". Archived from the original on 6 October 2014. Retrieved 8 October 2014.
- ↑ Almendrala A (6 October 2014). "Dallas Ebola Patient Receives Experimental Drug". The Huffington Post. Retrieved 8 October 2014.
- ↑ Berman M, Brown DL (8 October 2014). "Thomas Duncan, the Texas Ebola patient, has died". Washington Post. Retrieved 8 October 2014.
- ↑ Cohen E (7 October 2014). "Dallas Ebola patient waited nearly a week for experimental drug; family claims bias". CNN.
- ↑ Wilson T (22 October 2014). "Young and Healthy: How NBC News Freelancer Ashoka Mukpo Survived Ebola". NBC News.
- ↑ Loftus P (16 October 2014). "Chimerix to Conduct Ebola Drug Trial: Drug Company Gets FDA Approval to Start Trial Immediately in Infected Patients". The Wall Street Journal.
- ↑ Boseley S (17 February 2015). "Ebola: the race to find a cure". The Guardian. ISSN 0261-3077. Retrieved 16 March 2020.
- ↑ Giahyue JH (6 January 2015). "Trials of untested Ebola drugs begin in West Africa". Reuters. Archived from the original on 9 January 2015. Retrieved 6 January 2015.
- ↑ Kroll D (31 January 2015). "Chimerix Ends Brincidofovir Ebola Trials to Focus on Adenovirus and CMV". Forbes. Retrieved 31 January 2015.
- ↑ "Ebola drug trial in Liberia halted". Médecins Sans Frontières. 3 February 2015.
- ↑ Quenelle DC, Lampert B, Collins DJ, Rice TL, Painter GR, Kern ER (November 2010). "Efficacy of CMX001 against herpes simplex virus infections in mice and correlations with drug distribution studies". The Journal of Infectious Diseases. 202 (10): 1492–1499. doi:10.1086/656717. PMC 2957530. PMID 20923374.
- ↑ David Kroll (7 October 2014). "Chimerix's Brincidofovir Given To Dallas, Nebraska Ebola Patients". forbes.com.
Further reading
- Kern ER, Hartline C, Harden E, Keith K, Rodriguez N, Beadle JR, Hostetler KY (April 2002). "Enhanced inhibition of orthopoxvirus replication in vitro by alkoxyalkyl esters of cidofovir and cyclic cidofovir". Antimicrobial Agents and Chemotherapy. 46 (4): 991–995. doi:10.1128/aac.46.4.991-995.2002. PMC 127114. PMID 11897580.
External links
- "Brincidofovir". Drug Information Portal. U.S. National Library of Medicine.