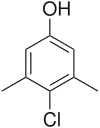
Chloroxylenol
 | |
| Names | |
|---|---|
| Systematic IUPAC name
4-Chloro-3,5-dimethylphenol[1] | |
Other names
| |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
Beilstein Reference |
1862539 |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| EC Number |
|
| KEGG | |
| MeSH | chloroxylenol |
PubChem CID |
|
| RTECS number |
|
| UNII | |
InChI
| |
SMILES
| |
| Properties | |
Chemical formula |
C8H9ClO |
| Molar mass | 156.61 g·mol−1 |
| Melting point | 114 to 116 °C (237 to 241 °F; 387 to 389 K) |
| log P | 3.377 |
| Acidity (pKa) | 9.76 |
| Basicity (pKb) | 4.24 |
| Pharmacology | |
| D08AE05 (WHO) | |
| Hazards | |
| GHS pictograms |  |
| GHS Signal word | Warning |
GHS hazard statements |
H302, H315, H317, H319 |
GHS precautionary statements |
P280, P305+351+338 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Chloroxylenol, also known as para-chloro-meta-xylenol (PCMX), is an antiseptic and disinfectant which is used for skin disinfection and cleaning surgical instruments.[2] It is also used within a number of household disinfectants and wound cleaners.[3] It is less effective than some other available agents.[4][5] It is available as a liquid.[2]
Side effects are generally few but can include skin irritation.[2][4] It may be used mixed with water or alcohol.[2] Chloroxylenol is most effective against gram-positive bacteria.[2] It works by disruption of the cell wall and stopping the function of enzymes.[5]
Chloroxylenol was first made in 1927.[6] It is on the World Health Organization's List of Essential Medicines.[7] The wholesale cost in the developing world is about US$2.00–6.20 per litre of 5% solution.[8] It is sold in a number of formulations and under a number of brand names, including Dettol.[3][9]
Uses
Chloroxylenol is used in hospitals and households for disinfection and sanitation. It is also commonly used in antibacterial soaps, wound-cleansing applications and household antiseptics such as Dettol liquid (to which it contributes its distinctive odor), cream and ointments.[10]
Side effects
Chloroxylenol is not significantly toxic to humans, is practically non-toxic to birds, and is moderately toxic to freshwater invertebrates. It is highly toxic to fish, cats, and some amphibians and should not be used around them.[11] It is a mild skin irritant and may trigger allergic reactions in some individuals.[12]
Humans
Excessive exposure to chloroxylenol has the potential for causing death. It can be poisonous when swallowed and even when it is unintentionally inhaled. A medical study in Hong Kong which analyzed 177 cases of Dettol ingestion that resulted in emergency department treatment (95% of which were intentional), concluded that "Dettol poisoning resulted in serious complications in 7% of patients, including death."[13]
Other animals
Chloroxylenol is toxic to many animals, especially cats.[14] Phenolic compounds are of particular concern because cats are unable to fully metabolize them. A cat may swallow the product by licking its paws after they have come into contact with it.
In Australia, chloroxylenol spray has been shown to be lethal to cane toads, an invasive species that was introduced from Hawaii as a result of bad judgment in 1935. It had been hoped that the amphibian would control the cane beetle but it became highly destructive within the ecosystem. Spraying the disinfectant at close range has been shown to cause rapid death to toads. It is not known whether the toxins are persistent or whether they harm other Australian flora and fauna.
Owing to concerns over potential harm to other Australian wildlife species, the use of chloroxylenol as an agent for pest control was banned in Western Australia by the Department of Environment and Conservation in 2011.[15]
History
Soon after it was created parachlorometaxylenol was then called PCMX, but this was thought to be a poor name and it was renamed Dettol. Then in 1932 it was marketed in Britain and in India. It had a white on green bottle with a white sword depicted.[16][17] It is sold, in the same style bottle, in Argentina and Uruguay to this day.[18]
Society and culture
A number of brand names are available. Chloroxylenol is the active ingredient in Dettol. It comprises 4.8% of Dettol's total admixture,[19] with the rest made up by pine oil, isopropanol, castor oil, soap and water.
References
- ↑ "Chloroxylenol". pubchem.ncbi.nlm.nih.gov. Archived from the original on 20 January 2021. Retrieved 4 November 2020.
- 1 2 3 4 5 World Health Organization (2009). Stuart MC, Kouimtzi M, Hill SR (eds.). WHO Model Formulary 2008. World Health Organization. p. 324. hdl:10665/44053. ISBN 9789241547659.
- 1 2 Griffiths, Christopher; Barker, Jonathan; Bleiker, Tanya; Chalmers, Robert; Creamer, Daniel (2016-02-29). Rook's Textbook of Dermatology, 4 Volume Set. John Wiley & Sons. p. 128.38. ISBN 9781118441176. Archived from the original on 2017-01-13.
- 1 2 Digison, MB (2007). "A review of anti-septic agents for pre-operative skin preparation". Plastic Surgical Nursing. 27 (4): 185–9, quiz 190–1. doi:10.1097/01.psn.0000306182.50071.e2. PMID 18165724.
- 1 2 Mahon, Connie R.; Lehman, Donald C.; Jr, George Manuselis (2014). Textbook of Diagnostic Microbiology (5 ed.). Elsevier Health Sciences. p. 67. ISBN 9780323292627. Archived from the original on 2017-01-13.
- ↑ Larson, E; Talbot, GH (August 1986). "An approach for selection of health care personnel handwashing agents". Infection Control. 7 (8): 419–24. doi:10.1017/s0195941700064663. PMID 3091524.
- ↑ World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- ↑ "Chloroxylenol". International Drug Price Indicator Guide. Archived from the original on 22 January 2018. Retrieved 8 December 2016.
- ↑ "Chloroxylenol - brand name list from Drugs.com". www.drugs.com. Archived from the original on 2017-08-28.
- ↑ Ascenzi, Joseph M. (1996). "Chloroxylenol: an old-new antimicrobial". Handbook of disinfectants and antiseptics. New York: M. Dekker. ISBN 978-0-8247-9524-5. Archived from the original on 2017-09-23.
- ↑ Dettol liquid at drugs.com Archived 2015-09-24 at the Wayback Machine
- ↑ K Verma, Ghanshyam; K Mahajan, Vikram; Shanker, Vinay; Ram Tegta, Geeta; Jindal, Nidhi; Minhas, Samridhi (2011). "Contact depigmentation following irritant contact dermatitis to chloroxylenol (dettol)". Indian J Dermatol Venereol Leprol. 77 (5): 612–4. doi:10.4103/0378-6323.84086. PMID 21860168.
- ↑ PK Lam; CK Chan; ML Tse; FL Lau (August 2012). "Dettol poisoning and the need for airway intervention" (PDF). Hong Kong Medical Journal. 18 (4): 270–275. PMID 22865169. Archived (PDF) from the original on 2 April 2015. Retrieved 29 March 2015.
- ↑ "Cats and poisons". icatcare.org. Archived from the original on 2015-08-31.
- ↑ Narelle Towie (23 May 2009). "Cane toad poison banned". Perth Now. Archived from the original on 15 January 2013. Retrieved 2 February 2013.
- ↑ Susan Pinto, Viveat (12 March 2015). "40 years ago...And now: Not just germs, Dettol fights rivals unabated". Business Standard India. www.business-standard.com. Archived from the original on 30 March 2017. Retrieved 12 July 2017.
- ↑ C.S.G. Krishnamacharyulu Rural Marketing: Text and Cases (2010), p. 407, at Google Books
- ↑ "Espadol Dettol CLOROXILENOL Antiséptico". Archived from the original on 2 February 2018. Retrieved 1 February 2018.
- ↑ "Summary of Product Characteristics / Dettol Liquid" (PDF). MHRA. 22 November 2010. Archived from the original (PDF) on 8 January 2015. Retrieved 9 November 2014.
External links
- "Chloroxylenol". Drug Information Portal. U.S. National Library of Medicine. Archived from the original on 2021-05-23. Retrieved 2020-05-17.