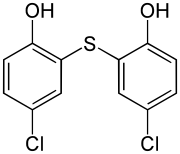
Fenticlor
 | |
| Clinical data | |
|---|---|
| Routes of administration | topical |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.002.336 |
| Chemical and physical data | |
| Formula | C12H8Cl2O2S |
| Molar mass | 287.15 g·mol−1 |
| Melting point | 174 °C (345 °F) |
Fenticlor (also spelled fentichlor) is an antibacterial and antifungal agent for topical use. It is an antimicrobial agent. It is also used in veterinary medicine.
Synthesis
It is prepared by the AlCl3-catalyzed reaction of 4-chlorophenol with sulfur dichloride.[1] It can also be prepared by chlorination of bis[2-hydroxyphenyl]sulfide.[2][3]
Safety
LD50 (rats, oral) = 3250 mg/kg.[1] It may cause photosensitivity[4]
References
- 1 2 Fiege H, Voges HW, Hamamoto T, Umemura S, Iwata T, Miki H, Fujita Y, Buysch HJ, Garbe D, Paulus W (2007). "Phenol Derivatives". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a19_313.
- ↑ DE 568944, Muth F, "Verfahren zur Darstellung von Bis-(halogen-oxyaryl)-sulfiden", issued 1931, assigned to I. G. Farben
- ↑ Dunning F, Dunning B, Drake WE (1931). "Preparation and Bacteriological Study of Some Symmetrical Organic Sulfides". Journal of the American Chemical Society. 53 (9): 3466. doi:10.1021/ja01360a035.
- ↑ Burry JN (June 1974). "Letter: Fenticlor, actinic reticuloid, and antihistamines". British Medical Journal. 2 (5918): 556–7. doi:10.1136/bmj.2.5918.556-c. PMC 1610938. PMID 4152080.
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.