Cinaciguat
 | |
| Clinical data | |
|---|---|
| Routes of administration | intravenous (?) |
| ATC code |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
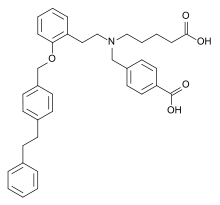
| Formula | C36H39NO5 |
| Molar mass | 565.710 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
Cinaciguat (BAY 58-2667) is an experimental drug for the treatment of acute decompensated heart failure.
Mechanism of action
Cinaciguat activates the soluble guanylate cyclase (sGC) which is a receptor for nitric oxide. This increases biosynthesis of cyclic GMP, resulting in vasodilation.[1]
See also
- Riociguat, another drug stimulating sGC, but with a different mechanism
- PDE5 inhibitors act further downstream in the nitric oxide signalling pathway, reducing cyclic GMP degradation.
References
- ↑ Schubert-Zsilavecz, M, Wurglics, M, Neue Arzneimittel 2009
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.