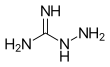
Pimagedine
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
2-Aminoguanidine | |||
Other names
| |||
| Identifiers | |||
CAS Number |
|||
3D model (JSmol) |
|||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.001.076 | ||
| KEGG | |||
PubChem CID |
|||
| UNII | |||
CompTox Dashboard (EPA) |
|||
InChI
| |||
SMILES
| |||
| Properties | |||
Chemical formula |
CH6N4 | ||
| Molar mass | 74.085 g/mol | ||
| Density | 1.72 g/ml | ||
| Boiling point | 261 °C (502 °F; 534 K) | ||
| log P | −1.475 | ||
| Related compounds | |||
Related compounds |
Guanidine | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Pimagedine, also known as aminoguanidine, is an investigational drug for the treatment of diabetic nephropathy that is no longer under development as a drug.[1] Pimagedine functions as an inhibitor of diamine oxidase and nitric oxide synthase. It acts to reduce levels of advanced glycation end products (AGEs) through interacting with 3-deoxyglucosone, glyoxal, methylglyoxal, and related dicarbonyls. These reactive species are converted to less reactive heterocycles by this condensation reaction.
History
Pimagedine was under development as a drug for kidney diseases by the pharmaceutical company Alteon (now known Synvista Therapeutics Inc.) that was founded in 1986.[2] In 1987, Alteon acquired a license to intellectual property relating to AGE inhibition from Rockefeller University.[3] In 1989, Alteon and Marion Merrell Dow Inc (MMD) entered into a joint development program for pimagedine.[4] In 1992, Alteon licensed a patent from Rockefeller University relating to the use of pimagedine to inhibit AGE formation.[3] In 1995, Hoechst AG (now Sanofi-Aventis) acquired MMD and subsequently terminated its agreement with Alteon, which led Alteon to stop clinical trials, which caused some controversy.[4] In 1997, Alteon and Genentech announced a collaboration under which Genentech would fund development of pimagedine and would have the rights to sell the drug if it would be approved.[5]
In March 1998, Alteon announced that it had been advised that it should discontinue its Phase III trial of pimagedine in non-insulin-dependent (type II) diabetes patients with overt nephropathy, after the trial's external safety monitoring committee found an increased risk of side effects in the treatment group.[6] In November 1998, Alteon announced that its Phase III trial for pimagedine as a treatment for end stage renal disease had failed to prove efficacy, which led Carl Gordon, a leading biotech analyst, to say: "It looks like pimagedine is probably finished."[7] In February, 1999, Genentech ended its collaboration with Alteon to develop pimagedine.[8] In April 1999 Alteon announced that it would cease development of pimagedine as a treatment for end stage renal disease but might consider continuing development in type 1 diabetic patients with overt nephropathy or progressive kidney disease.[9] Alteon's 2000, 2001, 2002 annual reports indicated that it was not running any clinical trials on pimagedine but was seeking co-development partners.[10][11][12] Alteon's 2003 and subsequent annual reports did not mention that Alteon was seeking partners for pimagedine,[13] which indicated that efforts to interest other companies and investors had failed and which signaled that commercial efforts to develop pimagedine as a drug were indeed finished.
Chemistry
Synthesis
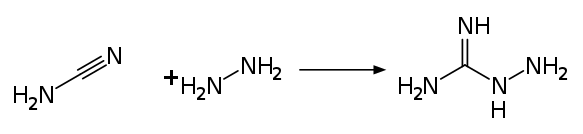
The industrial synthesis uses the reaction between cyanamide and hydrazine in aqueous solution.[14]
The compound can also be obtained from the reduction of nitroguanidine with zinc in acetic acid.[15]
Properties
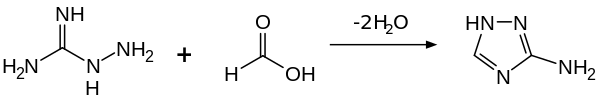
Aminoguanidine forms a colorless crystal that is soluble in water and ethanol. It is basic, producing salts when reacted with organic acids. With formic acid, a cyclization takes place to give 3-amino-1,2,4-triazole.[14]
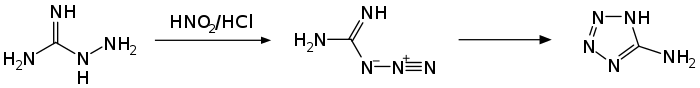
The compound reacts with nitrous acid in acidic medium to give 5-aminotetrazole via the intermediate guanylazide.[14] At neutral pH, the reaction leads to tetrazene.[16] Diazotization in acetic acid yields 1,3-di-(tetrazolyl)-triazene.[14]
References
- ↑ Thornalley, Paul J. (2003). "Use of aminoguanidine (Pimagedine) to prevent the formation of advanced glycation endproducts". Archives of Biochemistry and Biophysics. 419 (1): 31–40. doi:10.1016/j.abb.2003.08.013. PMID 14568006.
- ↑ Synvista Therapeutics Inc. Biocentury. Retrieved August 17, 2017.
- 1 2 "Alteon 10-K For the fiscal year ended December 31, 1996". Alteon via SEC Edgar. March 27, 1997.
- 1 2 Harry Keen; JH Fukker; G Menzinger (July 19, 1997). "Early closure of European Pimagedine trial". The Lancet. PlumX Metrics. 350 (9072): 214–215. doi:10.1016/S0140-6736(97)26029-0. PMID 9250200. S2CID 54316555.
- ↑ Barbara Marsh (January 3, 1998). "Biotech's New Watchword: Partnership". Los Angeles Times. Retrieved August 17, 2017.
- ↑ "Alteon May Drop Pimagedine In NIDDM". The Pharma Letter. March 19, 1998. Retrieved August 17, 2017.
- ↑ "Alteon Shares Plummet On Poor Pimagedine Test Results". San Diego Source. November 16, 1998. Retrieved August 17, 2017.
- ↑ http://business.globe24h.com/sec/001/06/060000/0000060271.shtml
- ↑ "Alteon's pimagedine fails primary endpoint". The Pharma Letter. April 12, 1999. Retrieved August 17, 2017.
- ↑ https://www.sec.gov/Archives/edgar/data/878903/0000893220-00-000381.txt
- ↑ https://www.sec.gov/Archives/edgar/data/878903/000089322001000240/0000893220-01-000240.txt
- ↑ https://www.sec.gov/Archives/edgar/data/878903/000089322002000222/0000893220-02-000222.txt
- ↑ https://www.sec.gov/Archives/edgar/data/878903/000089322003000272/0000893220-03-000272.txt
- 1 2 3 4 Güthner, Thomas; Mertschenk, Bernd; Schulz, Bernd (2006), "Guanidine and Derivatives", Ullmann's Encyclopedia of Industrial Chemistry, American Cancer Society, doi:10.1002/14356007.a12_545.pub2, ISBN 978-3-527-30673-2
- ↑ Smith, G. B. L.; Anzelmi, Edward (1935-12-01). "Reduction of Nitroguanidine. III. Synthesis of Aminoguanidine1". Journal of the American Chemical Society. 57 (12): 2730. doi:10.1021/ja01315a510. ISSN 0002-7863.
- ↑ Patinkin, Seymour H.; Horwitz, Jerome P.; Lieber, Eugene (1955-02-01). "The Structure of Tetracene1,2". Journal of the American Chemical Society. 77 (3): 562–567. doi:10.1021/ja01608a014. ISSN 0002-7863.