Coarctation of the aorta
| Coarctation of the aorta | |
|---|---|
| Other names: Aortic coarctation , aortic narrowing | |
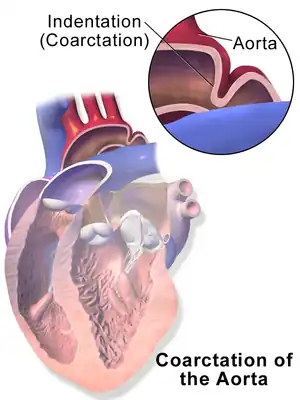 | |
| Illustration depicting coarctation of the aorta | |
| Specialty | Cardiac surgery |
| Symptoms | Newborn: Trouble breathing, sweating, irritable[1] Later: High blood pressure in arms[1] |
| Complications | Coronary artery disease, aortic aneurysm, heart failure, cerebral vascular disease[2][1] |
| Usual onset | Present at birth, though symptoms may develop later[1] |
| Duration | Life-long[2] |
| Causes | Unknown[2] |
| Risk factors | Family history, Turner syndrome[3] |
| Diagnostic method | Based on blood pressure, confirmed by medical imaging[2][3] |
| Differential diagnosis | Myocarditis, aortic dissection, hypoplastic left heart syndrome, sepsis[2] |
| Treatment | Surgery (open or endovascular)[2] |
| Medication | Prostaglandin E1[2] |
| Frequency | 4 per 10,000,[1] male:female=2:1[3] |
Coarctation of the aorta (CoA, CoAo) is a narrowing of the aorta, most commonly just beyond the exit of the left subclavian artery.[3] While present at birth, onset of symptoms may not occur until 1 to 2 weeks after birth with trouble breathing or later in life with high blood pressure in the arms.[2][1] Complications, without treatment, can include coronary artery disease, aortic aneurysm, heart failure, and cerebral vascular disease.[2][1]
The cause is generally unclear.[2] Risk factors include family history and Turner syndrome.[2] The underlying mechanism is though to usually involve closure of the ductus arteriosus as it becomes the ligamentum arteriosum.[2] Diagnosis is generally suspected based on checking the blood pressure in all four limbs and confirmed by echocardiography.[2][3]
In newborns prostaglandin E1 may be used to temporize the condition.[2] Surgery, either open or via a blood vessel, may than be carried out.[2] Ongoing medications to manage blood pressure may be required.[1] Following surgery there is a risk of reoccurrence.[2]
About 4 per 10,000 newborns are affected.[1] It accounts for around 6% of all heart disease present at birth.[3] Males are affected twice as common as females.[3] The condition was first described by Giovanni Battista Morgagni in 1760.[4]
Signs and symptoms
In mild cases, children may show no signs or symptoms at first and their condition may not be diagnosed until later in life.[3] Some children born with coarctation of the aorta have additional heart defects, such as aortic stenosis, ventricular septal defect, patent ductus arteriosus or mitral valve abnormalities.
Coarctation is about twice as common in boys as it is in girls. It is common in girls who have Turner syndrome.
Symptoms may be absent with mild narrowings (coarctation). When present, they include breathing difficulties, poor appetite or trouble feeding, and failure to thrive. Later on, children may develop symptoms related to problems with blood flow and an enlarged heart. They may experience dizziness or shortness of breath, fainting or near-fainting episodes, chest pain, abnormal tiredness or fatigue, headaches, or nosebleeds. They have cold legs and feet or have pain in their legs with exercise (intermittent claudication).
In cases of more severe coarctations, babies may develop serious problems soon after birth because not enough blood can get through the aorta to the rest of their body. Arterial hypertension in the arms with low blood pressure in the lower extremities is classic. In the lower extremities, weak pulses in the femoral arteries and arteries of the feet are found.
The coarctation typically occurs after the left subclavian artery. However, if situated before it, blood flow to the left arm is compromised and asynchronous or radial pulses of different "strength" may be detected (normal on the right arm, weak or delayed on the left), termed radio-radial delay. In these cases, a difference between the normal radial pulse in the right arm and the delayed femoral pulse in the legs (either side) may be apparent, whilst no such delay would be appreciated with palpation of both delayed left arm and either femoral pulses. On the other hand, a coarctation occurring after the left subclavian artery will produce synchronous radial pulses, but radio-femoral delay will be present under palpation in either arm (both arm pulses are normal compared to the delayed leg pulses).
Diagnosis
With imaging, resorption of the lower part of the ribs may be seen, due to increased blood flow over the neurovascular bundle that runs there. Prestenotic dilatation of the aortic arch and left subclavian artery, as well as indentation at the site of coarctation results in a classic 'figure 3 sign' on x-ray. The characteristic bulging of the sign is caused by dilatation of the aorta due to an indrawing of the aortic wall at the site of cervical rib obstruction, with consequent poststenotic dilatation. This physiology results in the '3' image for which the sign is named.[5][6][7] When the esophagus is filled with barium, a reverse 3 or E sign is often seen and represents a mirror image of the areas of prestenotic and poststenotic dilatation.[8]
Coarctation of the aorta can be accurately diagnosed with magnetic resonance angiography. In teenagers and adults echocardiograms may not be conclusive.
The severity of coarctation of the aorta can be rated by a combination of the smallest aortic cross-sectional area of the aorta (adjusted for body surface area) as measured by 3D-rendered contrast MRI, as well as mean heart rate–corrected flow deceleration in the descending aorta as measured by phase contrast magnetic resonance imaging.[9]
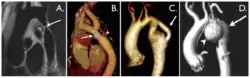 Aortic coarctation using different imaging techniques[10]
Aortic coarctation using different imaging techniques[10]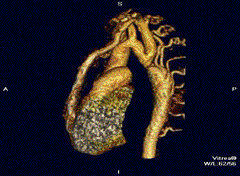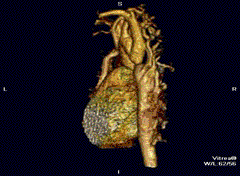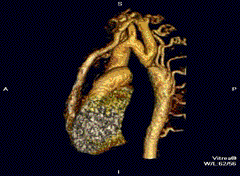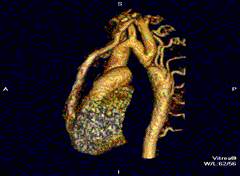 Coarctation of the aorta
Coarctation of the aorta.jpg.webp) Coarctation of the aorta
Coarctation of the aorta.jpg.webp) Coarctation of the aorta
Coarctation of the aorta
Classification
There are three types of aortic coarctations:[11]
- Preductal coarctation: The narrowing is proximal to the ductus arteriosus. Blood flow to the aorta that is distal to the narrowing is dependent on the ductus arteriosus; therefore severe coarctation can be life-threatening. Preductal coarctation results when an intracardiac anomaly during fetal life decreases blood flow through the left side of the heart, leading to hypoplastic development of the aorta. This is the type seen in approximately 5% of infants with Turner syndrome.[12][13]
- Ductal coarctation: The narrowing occurs at the insertion of the ductus arteriosus. This kind usually appears when the ductus arteriosus closes.
- Postductal coarctation: The narrowing is distal to the insertion of the ductus arteriosus. Even with an open ductus arteriosus, blood flow to the lower body can be impaired. This type is most common in adults. It is associated with notching of the ribs (because of collateral circulation), hypertension in the upper extremities, and weak pulses in the lower extremities. Postductal coarctation is most likely the result of the extension of a muscular artery (ductus arteriosus) into an elastic artery (aorta) during fetal life, where the contraction and fibrosis of the ductus arteriosus upon birth subsequently narrows the aortic lumen.[14]
Aortic coarctation and aortic stenosis are both forms of aortic narrowing. In terms of word root meanings, the names are not different, but a conventional distinction in their usage allows differentiation of clinical aspects. This spectrum is dichotomized by the idea that aortic coarctation occurs in the aortic arch, at or near the ductus arteriosis, whereas aortic stenosis occurs in the aortic root, at or near the aortic valve. This naturally could present the question of the dividing line between a postvalvular stenosis and a preductal coarctation; nonetheless, the dichotomy has practical use, as most defects are either one or the other.
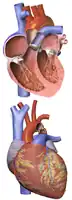 Illustration showing a heart with a coarctation of the aorta
Illustration showing a heart with a coarctation of the aorta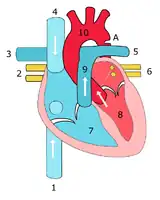 Sketch showing heart with coarctation of the aorta. A: Coarctation (narrowing) of the aorta. 1:Inferior vena cava, 2:Right pulmonary veins, 3: Right pulmonary artery, 4:Superior vena cava, 5:Left pulmonary artery, 6:Left pulmonary veins, 7:Right ventricle, 8:Left ventricle, 9:Pulmonary artery, 10:Aorta
Sketch showing heart with coarctation of the aorta. A: Coarctation (narrowing) of the aorta. 1:Inferior vena cava, 2:Right pulmonary veins, 3: Right pulmonary artery, 4:Superior vena cava, 5:Left pulmonary artery, 6:Left pulmonary veins, 7:Right ventricle, 8:Left ventricle, 9:Pulmonary artery, 10:Aorta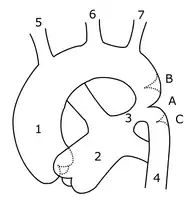 Schematic drawing of alternative locations of a coarctation of the aorta, relative to the ductus arteriosus. A: Ductal coarctation, B: Preductal coarctation, C: Postductal coarctation. 1: Aorta ascendens, 2: Arteria pulmonalis, 3: Ductus arteriosus, 4: Aorta descendens, 5: Truncus brachiocephalicus, 6: Arteria carotis communis sinistra, 7: Arteria subclavia sinistra
Schematic drawing of alternative locations of a coarctation of the aorta, relative to the ductus arteriosus. A: Ductal coarctation, B: Preductal coarctation, C: Postductal coarctation. 1: Aorta ascendens, 2: Arteria pulmonalis, 3: Ductus arteriosus, 4: Aorta descendens, 5: Truncus brachiocephalicus, 6: Arteria carotis communis sinistra, 7: Arteria subclavia sinistra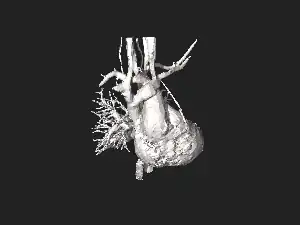 3D model of coarctation of aorta
3D model of coarctation of aorta
Prevention
Unfortunately, coarctations can not be prevented because they are usually present at birth. The best thing for patients who are affected by coarctations is early detection. Some signs that can lead to a coarctation have been linked to pathologies such as Turner syndrome, bicuspid aortic valve, and other family heart conditions.
Treatment
In adults and children found to have coarctation, treatment is conservative if asymptomatic, but may require surgical resection of the narrow segment if there is arterial hypertension. The first operations to treat coarctation were carried out by Clarence Crafoord in Sweden in 1944.[15] In some cases angioplasty can be performed to dilate the narrowed artery, with or without the placement of a stent graft.
For fetuses at high risk for developing coarctation, a novel experimental treatment approach is being investigated, wherein the mother inhales 45% oxygen three times a day (3 x 3–4 hours) beyond 34 weeks of gestation. The oxygen is transferred via the placenta to the fetus and results in dilatation of the fetal lung vessels. As a consequence, the flow of blood through the fetal circulatory system increases, including that through the underdeveloped arch. In suitable fetuses, marked increases in aortic arch dimensions have been observed over treatment periods of about two to three weeks.[16]
The long term outcome is very good. Some patients may, however, develop narrowing (stenosis) or dilatation at the previous coarctation site. All patients with unrepaired or repaired aortic coarctation require follow up in specialized Congenital Heart Disease centers.
Complications of surgery
Surgical treatment involves resection of the stenosed segment and re-anastomosis. Two complications specific to this surgery are left recurrent nerve palsy and chylothorax, as the recurrent laryngeal nerve and thoracic duct are in the vicinity. Chylothorax is a troublesome complication and is usually managed conservatively by adjusting the diet to eliminate long-chain fatty acids and supplementing medium-chain triglycerides. When conservative management fails surgical intervention is then most often required.[17] Fluorescein dye can aid in the localisation of chyle leak.[18]
Prognosis
Side effects
Previously, hypertension was defined as a blood pressure of 140/90 mm Hg but has since been revised by the American College of Cardiology/American Heart Association Task Force to a blood pressure of 130/80 mm Hg or higher in adults.[19] This is a severe problem for the heart and can cause many other complications. In a study of 120 coarctation repair recipients done in Groningen, The Netherlands, twenty-nine patients (25%) experienced hypertension in the later years of life due to the repair. While hypertension has many different factors that lead to this stage of blood pressure, people who have had a coarctation repair — regardless of the age at which the operation was performed — are at much higher risk than the general public of hypertension later in life. Undetected chronic hypertension can lead to sudden death among coarctation repair patients, at higher rates as time progresses.
Angioplasty is a procedure done to dilate an abnormally narrow section of a blood vessel to allow better blood flow. This is done in a cardiac catheterization laboratory. Typically taking two to three hours, the procedure may take longer but usually patients are able to leave the hospital the same day. After a coarctation repair 20-60% of infant patients may experience reoccurring stenosis at the site of the original operation. This can be fixed by either another coarctectomy.[20]
Coronary artery disease (CAD) is a major issue for patients who have undergone a coarctation repair. Many years after the procedure is done, heart disease not only has an increased chance of affecting coarctation patients, but also progresses through the levels of severity at an alarmingly increased rate. In one study, one fourth of the patients who experienced a coarctation later died of heart disease, some at a relatively young age.[21][22]
Clinical criteria are used in most studies when defining recurrence of coarctation (recoarctation) when blood pressure is at a difference of >20 mmHg between the lower and upper limbs. This procedure is most common in infant patients and is uncommon in adult patients. 10.8% of infant patients underwent recoarctations at less than two years of age while another 3.1% of older children received a recoarctation.[23]
People who have had a coarctation of the aorta are likely to have bicuspid aortic valve disease. Between 20% and 85% of patients are affected with this disease. Bicuspid aortic valve disease is a big contributor to cardiac failure, which in turn makes up roughly 20% of late deaths to coarctation patients.[23]
Follow-up
Because of the risk of recoarctation and late hypertension, check-ups are needed once a year or less frequently depending on the individual case. It is important to visit the cardiologist on a regular basis. Depending on the severity of the patient's condition, which is evaluated on a case-by-case level, visiting a cardiologist can be a once a year or less frequent surveillance check up. Keeping a regular schedule of appointments with a cardiologist after a coarctation procedure is complete helps increase the chances of optimal health for the patients. Nowadays, life expectancy is considered normal given the repair was successfully done in early childhood. Treatment of recoarctation is usually successfully done without the need for open heart surgery. Recoarctation is increasingly less common in the modern era. Late hypertension does also seem to be much less of a problem if the coarctation repair was performed within the first 5 years of life. Life expectancy and quality of life are therefore the same or very close to that of the normal population, but check ups are recommended so that those few percent who need further treatment get it in time.[24]
History
The condition was first described by Giovanni Battista Morgagni in 1760.[4]
It was also discovered in Julia the daughter of the French poet Alphonse de Lamartine after the autopsy in 1832 in Beirut,[4] the reference manuscript still exists in one of the Maronite monasteries in Mount Lebanon.
The word coarctation means "pressing or drawing together; narrowing".
References
- 1 2 3 4 5 6 7 8 9 "Congenital Heart Defects - Facts about Coarctation of the Aorta | CDC". Centers for Disease Control and Prevention. 22 November 2019. Archived from the original on 19 January 2021. Retrieved 20 January 2021.
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 Law, MA; Tivakaran, VS (January 2020). "Coarctation of the Aorta". PMID 28613663.
{{cite journal}}: Cite journal requires|journal=(help) - 1 2 3 4 5 6 7 8 Mussa, Shafi; Anderson, David R.; Kumar, T. K. Susheel; Knott-Craig, Christopher J. (2020). "96. Coarctation of the aorta". In Raja, Shahzad G. (ed.). Cardiac Surgery: A Complete Guide. Switzerland: Springer. pp. 875–883. ISBN 978-3-030-24176-6. Archived from the original on 2022-10-15. Retrieved 2022-10-14.
- 1 2 3 Yeh, Doreen DeFaria; Bhatt, Ami (2018). Adult Congenital Heart Disease in Clinical Practice. Springer. p. 218. ISBN 978-3-319-67420-9. Archived from the original on 2021-08-28. Retrieved 2021-02-18.
- ↑ Brant, William E.; Helms, Clyde A., eds. (2012). "Coarctation of the aorta". Fundamentals of Diagnostic Radiology. Lippincott Williams & Wilkins. p. 1172. ISBN 978-1-60831-911-4.
{{cite book}}:|access-date=requires|url=(help);|archive-url=requires|url=(help); Unknown parameter|chapterurl=ignored (help) - ↑ Blecha, Matthew J. (August 30, 2005). General Surgery ABSITE and Board Review. Pearls of Wisdom. McGraw-Hill. ISBN 978-0-07-146431-4.
- ↑ Pregerson, Brady (October 1, 2006). Quick Essentials: Emergency Medicine (2nd ed.). ED Insight Books. ISBN 978-0-9761552-7-0.
- ↑ Aortic Coarctation Imaging at eMedicine
- ↑ Nielsen, J. C. (2005). "Magnetic Resonance Imaging Predictors of Coarctation Severity". Circulation. 111 (5): 622–628. doi:10.1161/01.CIR.0000154549.53684.64. ISSN 0009-7322. PMID 15699283.
- ↑ Ntsinjana, Hopewell N; Hughes, Marina L; Taylor, Andrew M (2011). "The Role of Cardiovascular Magnetic Resonance in Pediatric Congenital Heart Disease". Journal of Cardiovascular Magnetic Resonance. 13: 51. doi:10.1186/1532-429X-13-51. PMC 3210092. PMID 21936913.
- ↑ Valdes-Cruz, Lilliam M.; Cayre, Raul O., eds. (1999). Echocardiographic Diagnosis of Congenital Heart Disease: An Embryologic and Anatomic Approach. Philadelphia: Lippincott Williams & Wilkins. ISBN 978-0-7817-1433-4.
- ↑ Cotran, R.; V. Kumar; N. Fausto (2005). Robbins Pathologic Basis of Disease (7th ed.). W.B. Saunders. ISBN 978-0-8089-2302-2.
{{cite book}}: Unknown parameter|last-author-amp=ignored (help) - ↑ Völkl, Thomas M. K.; Degenhardt, Karin; Koch, Andreas; Simm, Diemud; Dörr, Helmuth G.; Singer, Helmut (2005). "Cardiovascular anomalies in children and young adults with Ullrich-Turner syndrome-the erlangen experience". Clinical Cardiology. 28 (2): 88–92. doi:10.1002/clc.4960280209. PMC 6654047. PMID 15757080.
- ↑ Surgical Approach to Coarctation of the Aorta and Interrupted Aortic Arch at eMedicine
- ↑ Radegran, Kjell (2003). "The Early History of Cardiac Surgery in Stockholm". Journal of Cardiac Surgery. 18 (6): 564–72. doi:10.1046/j.0886-0440.2003.02071.x. PMID 14992112. S2CID 40925549.
- ↑ Kohl, T; Tchatcheva, K; Stressig, R; Geipel, A; Heitzer, S; Gembruch, U (2008). "Maternal hyperoxygenation in late gestation promotes rapid increase of cardiac dimensions in fetuses with hypoplastic left hearts with intrinsically normal or slightly abnormal aortic and mitral valves". Ultraschall in der Medizin. 29 (S 2). doi:10.1055/s-2008-1080778.
- ↑ {http://www.ctsnet.org/article/ligation-thoracic-duct-chylothorax}%5B%5D
- ↑ Mathew, Thomas; Idhrees, Mohammed; Misra, Satyajeet; Menon, Sabarinath; Dharan, Baiju Sasi; Karunakaran, Jayakumar (May 2015). "Intraoperative Identification of Chyle Leak During Coarctation Repair Using Fluorescein Dye". The Annals of Thoracic Surgery. 99 (5): 1827. doi:10.1016/j.athoracsur.2014.12.090. PMID 25952224.
- ↑ Whelton, Paul K.; Carey, Robert M.; Aronow, Wilbert S.; Casey, Donald E.; Collins, Karen J.; Himmelfarb, Cheryl Dennison; DePalma, Sondra M.; Gidding, Samuel; Jamerson, Kenneth A. (2017-01-01). "2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines". Hypertension. 71 (6): e13–e115. doi:10.1161/hyp.0000000000000065. ISSN 0194-911X. PMID 29133356.
- ↑ Beekman, Robert H.; Rocchini, Albert P.; Behrendt, Douglas M.; Bove, Edward L.; Dick, Macdonald; Crowley, Dennis C.; Rebecca Snider, A.; Rosenthal, Amnon (1986). "Long-term outcome after repair of coarctation in infancy: Subclavian angioplasty does not reduce the need for reoperation". Journal of the American College of Cardiology. 8 (6): 1406–11. doi:10.1016/s0735-1097(86)80314-x. PMID 2946743.
- ↑ Cohen, M.; Fuster, V.; Steele, P. M.; Driscoll, D.; McGoon, D. C. (1989). "Coarctation of the aorta. Long-term follow-up and prediction of outcome after surgical correction". Circulation. 80 (4): 840–5. doi:10.1161/01.CIR.80.4.840. PMID 2791247.
- ↑ Di Salvo, G; Castaldi, B; Baldini, L; Gala, S; del Gaizo, F; D'Andrea, A; Limongelli, G; D'Aiello, A F; Scognamiglio, G; Sarubbi, B; Pacileo, G; Russo, M G; Calabrò, R (2011). "Masked hypertension in young patients after successful aortic coarctation repair: impact on left ventricular geometry and function". Journal of Human Hypertension. 25 (12): 739–45. doi:10.1038/jhh.2010.118. PMID 21228825.
- 1 2 Giuffre, Michael; Ryerson, Lindsay; Chapple, Denise; Crawford, Susan; Harder, Joyce; Leung, Alexander K. C. (2005). "Nonductal dependent coarctation: a 20-year study of morbidity and mortality comparing early-to-late surgical repair". Journal of the National Medical Association. 97 (3): 352–6. PMC 2568624. PMID 15779499.
- ↑ Celermajer, DS; Greaves, K (2002). "Survivors of coarctation repair: fixed but not cured". Heart. 88 (2): 113–4. doi:10.1136/heart.88.2.113. PMC 1767208. PMID 12117824.
External links
- Coarctation of the Aorta Archived 2014-10-14 at the Wayback Machine - Stanford Children's Health
- Aortic Coarctation information from Seattle Children's Hospital Heart Center
| Classification | |
|---|---|
| External resources |