Hypoplastic left heart syndrome
| Hypoplastic left heart syndrome | |
|---|---|
| Other names: Cyanotic heart disease - hypoplastic left heart[1] | |
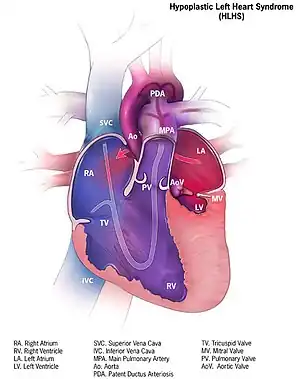 | |
| Illustration of heart suffering from hypoplastic left heart syndrome | |
| Specialty | Cardiac surgery |
| Symptoms | Shortness of breath, bluish skin[2] |
| Usual onset | Present at birth[3] |
| Causes | Usually unknown[2] |
| Diagnostic method | Ultrasound[3] |
| Differential diagnosis | Coarctation of the aorta, aortic stenosis, Shone's syndrome[3] |
| Treatment | Prostaglandin E1, surgery, comfort care[3] |
| Prognosis | High risk of disability or death[3] |
| Frequency | 1 in 5,000 newborns[3] |
Hypoplastic left heart syndrome (HLHS) is a congenital heart defect in which the left side of the heart is severely underdeveloped.[3] It may affect the left ventricle, aorta, aortic valve, and mitral valve.[3] While the baby may appear normal in the first few days of life, they than rapidly develop trouble breathing.[2] Other symptoms may include bluish skin with oxygen saturations between 75 and 85%.[3]
The cause is generally unknown.[2] It is associated with a number of genetic syndromes including Turner, DiGeorge, and Down.[3] Diagnosis is generally by prenatal ultrasound.[3]
Initial treatment is with prostaglandin E1 or comfort care.[3] A procedure to make a hole in the atrial septum may also be required.[3] This may be followed by a set of three surgeries.[3] ECMO may be used to bridge the baby until surgery.[3] Without treatment children die within the first week of life.[3] With treatment about 66% live to at least 5 years of age.[3]
HLHS is rare, affecting about 1 in 5,000 newborns.[3] It makes up about 3% of cases of congenital heart disease.[3] It was first described in 1952 by Lev.[4] Among cases diagnosed before birth 12% to 48% of parents elect to abort the pregnancy.[3]
Signs and symptoms
Closing of the ductus arteriosus in a heart that is severely underdeveloped on the left results in cyanosis and respiratory distress which can progress to cardiogenic shock and death. The first symptoms are cyanosis that does not respond to oxygen administration or poor feeding. Peripheral pulses may be weak and extremities cool to the touch.[5]
HLHS often co-occurs with low birth weight and premature birth.[5]
In neonates with a small atrial septal defect, termed "restrictive", there is inadequate mixing of oxygenated and deoxygenated blood. These neonates quickly decompensate and develop acidosis and cyanosis.[5]
On EKG, right axis deviation and right ventricular hypertrophy are common, but not indicative of HLHS. Chest x-ray may show a large heart (cardiomegaly) or increased pulmonary vasculature. Neonates with HLHS do not typically have a heart murmur, but in some cases, a pulmonary flow murmur or tricuspid regurgitation murmur may be audible.[5]
Co-occurring tricuspid regurgitation or right ventricular dysfunction can cause hepatomegaly to develop.[5]
Causes
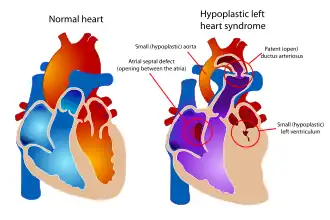
There is no known cause in the majority of HLHS cases.[6] Some cases may have a genetic component, as HLHS has been shown to be heritable and associated with specific gene mutations.[7][8]
Not all, but some, cases of aortic stenosis in a fetus can put stress on the left ventricle in utero, that can eventually lead to decreased perfusion and stop the growth of the left ventricle. [9]
Environmental
In a retrospective analysis affected newborns were more likely to be born in summer months, suggesting that seasonality and environmental factors may play a role.[10]
Genetics
Genetic loci associated with HLHS include GJA1 (connexin 43), HAND1, NKX2.5, 10q22, and 6q23.[11][12][13] There is a slight risk of recurrence in future pregnancies, estimated to be 2-4%, which increases to 25% in families with two affected children.[6] This is thought to be mediated by genetic mutations with incomplete penetrance.[11]
HLHS is also associated with several genetic syndromes, including trisomy 13 (Patau syndrome), trisomy 18 (Edwards syndrome), partial trisomy 9, Turner's syndrome (XO), Jacobsen syndrome (11q deletion syndrome), Holt-Oram syndrome, and Smith-Lemli-Opitz syndrome.[11][12]
Risk factors
Presence of a cystic hygroma increases the risk.[14]
Pathophysiology
At birth, the ductus arteriosus is still open, and there is higher than normal resistance to blood flow in the lungs. This allows for adequate oxygenation via mixing between the atria and a normal appearance at birth. When the ductus begins to close and pulmonary vascular resistance decreases, blood flow through the ductus is restricted and flow to the lungs is increased. [5]
In typical anatomy, the left side of the heart receives oxygen-rich blood from the lungs and pumps it to the rest of the body. In people with HLHS, the aorta and left ventricle are underdeveloped (beginning in utero),[15] and the aortic and mitral valves are either too small to allow sufficient blood flow or are atretic (closed) altogether.[16] As blood returns from the lungs to the left atrium, it cannot be pumped to the rest of the body by the left ventricle. The neonate is reliant on blood flowing through an atrial septal defect to mix oxygenated and deoxygenated blood, and on a patent ductus arteriosus to allow blood to reach the aorta and the systemic circulation via the right ventricle. This is what defines HLHS as a "single ventricle" defect.[11]
Due to the underdevelopment of the left side of the heart in utero, the increased afterload causes hypertension of the left atrium, pulmonary edema, and therefore lung damage to the fetus before birth. [9]
Diagnosis
Hypoplastic left heart syndrome can be diagnosed prenatally or after birth via echocardiography. Typical findings include a small left ventricle and aorta, abnormalities of the mitral and aortic valves, retrograde flow in the transverse arch of the aorta, and left-to-right flow between the atria. It is often recognized during the second trimester of pregnancy, between 18 and 24 weeks' gestation.[5]
Management
Medical
Without life-prolonging interventions, HLHS is fatal, but with intervention, an infant may survive. A cardiothoracic surgeon may perform a series of operations or a full heart transplant. While surgical intervention has emerged as the standard of care in the United States, other national health systems, notably in France, approach diagnosis of HLHS in a more conservative manner, with an emphasis on termination of pregnancy or compassionate care after delivery.[17]
Before surgery, the ductus must be kept open to allow blood-flow using medication containing prostaglandin. Air with less oxygen than normal is used for infants with hypoplastic left heart syndrome. These low oxygen levels increases the pulmonary vascular resistance (PVR) and thus improve blood flow to the rest of the body due to the greater pressure difference between the lungs and body. Achieving oxygen levels below atmosphere requires the use of inhaled nitrogen.[18] Nitric oxide is a potent pulmonary vasodilator, and thus reduces PVR and improves venous return. Any factor that increases PVR will impede right sided flow.[19][20]
Surgical
Surgical operations to assist with hypoplastic left heart are complex and need to be individualized for each patient. A cardiologist must assess all medical and surgical options on a case-by-case basis.
Currently, infants undergo either the staged reconstructive surgery (Norwood or Sano procedure within a few days of birth, Glenn or Hemi-Fontan procedure at 3 to 6 months of age, and the Fontan procedure at 1 1/2 to 5 years of age) or cardiac transplantation.[21] Current expectations are that 70% of those with HLHS may reach adulthood.[12] Many studies show that the higher the volume (number of surgeries performed) at a hospital, the lower the mortality (death) rate.[22][23] Factors that increase an infant's risk include lower birth weight, additional congenital anomalies, a genetic syndrome or those with a highly restrictive atrial septum.[24]) For patients without these additional risk factors, 5 year survival now approaches 80%.[24] Studies show that about 75% of those children who survive surgery show developmental delays in one or more areas, such as motor, cognitive, or language impairments, with about a third of single-ventricle children without a genetic syndrome having significant impairments.[25] Current research focuses on charting the connections between neurodevelopment injuries, surgical and intensive care procedures, and genetic susceptibility with the goal of modifying interventions that impair neurodevelopmental and psychosocial outcomes.[26] An alternative to the traditional Norwood is the Hybrid procedure.[27]
Some physicians offer compassionate care, instead of the surgeries, which results in the child's death, usually within 2 weeks of birth. Compassionate care is overseen by a physician, and may be carried out either in the hospital or at home. However, due to the vast improvement of surgical intervention, with many hospitals achieving over 90% survival, there is debate on whether or not compassionate care should still be offered to families.[28] A study in 2003 concluded that a selection of physicians who are experts in the care of children with HLHS were evenly split when asked what they would do if their own children were born with HLHS, with 1/3 stating that they would choose surgery, 1/3 stating that they would choose palliative (compassionate) treatment without surgery, and 1/3 stating that they are uncertain which choice they would make.[29]
The three-stage procedure is a palliative procedure (not a cure), as the child's circulation is made to work with only two of the heart's four chambers.
Norwood procedure
The first step is the Norwood procedure.[30] In this procedure, the right ventricle is used to pump blood into the systemic circulation. Since the right ventricle is no longer directly pumping blood to the lungs, a shunt is required in order to pass deoxygenated blood through the lungs. Either the subclavian artery can be connected to the pulmonary circulation (Blalock-Taussig shunt), or a shunt is made directly from the right ventricle to the pulmonary circulation (Sano shunt). The narrow aorta is enlarged using a patch to improve blood flow to the body.[31]
During this time the baby may be medically fragile and have feeding problems because the heart is working very hard. There is a considerable degree of venous mixing in the right ventricle, leading to lower oxygenation saturation. In addition, both the Blalock-Taussig and the Sano shunts expose the lungs to systemic arterial pressures, leading to long-term pulmonary hypertension and eventually heart failure.[30]
Hybrid procedure
The Hybrid procedure may be used in place of the Norwood.[30][32][33] The Hybrid procedure does not necessitate the use of heart-lung bypass or performing a sternotomy. Instead of a six-hour surgery, the Hybrid typically takes one to two hours. In this procedure, a stent is placed in the ductus arteriosus to maintain its patency, and bands are placed over both the left and right pulmonary artery branches to limit pressure and over-circulation to the lungs.[34] Outcomes with the Hybrid approach are comparable to those with the Norwood.[35]
Glenn procedure
The second stage—the bidirectional Glenn or Hemi-Fontan (see also Kawashima procedure)—relieves some of the problems introduced by Stage I palliation.[30] In this operation, the superior vena cava is ligated from the heart and connected to the pulmonary circulation. At this time, the Blalock-Taussig or Sano shunt is taken down. The lungs are no longer exposed to systemic arterial pressures, but much lower venous pressures. Although venous blood from the upper half of the body is no longer mixing with oxygenated blood in the right ventricle, there is still venous mixing from the lower half of the body, leading to some degree of oxygen desaturation.[30]
Fontan procedure
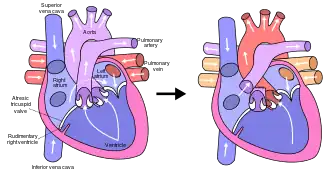
The final procedure, the Fontan procedure, completes the repair of the hypoplastic left heart.[30] Although there are several variations, the functional effect is to redirect venous blood from the lower body (through the inferior vena cava) away from the right atrium to the pulmonary artery. This should eliminate any mixing of oxygenated and deoxygenated blood in the right ventricle. The right ventricle performs the traditional job of the left, supplying the body with oxygenated blood, while the passive systemic venous pressure performs the traditional job of the right, passing deoxygenated blood to the lungs.[30]
Fetal surgery
Interventions performed during fetal development are under investigation. In fetuses with hypoplastic left ventricles and an intact interatrial septum, percutaneous atrial septostomy has been attempted.[14]
Prognosis
95% of untreated infants with HLHS die in the first weeks of life.[5]
Early survival has improved since the introduction of the Norwood procedure.[12] Since there are no long-term studies of HLHS adults, statistics are usually derived from post-Fontan patients; it is estimated that 70% of HLHS patients may reach adulthood.[12]
Prognosis is dependent upon the health of the child, as there is an increased demand on respiratory and heart rate in infants during common childhood illnesses. This fragile population has little cardiac reserve to accommodate these demands and provide hemodynamic stability during illnesses. [36]
Children with HLHS and other comparable single-ventricle conditions, as a group, have poorer neurodevelopmental outcomes than their healthy peers. Deficits in language, executive functioning, and higher rates of anxiety and depression disorders have been demonstrated.[37] Some of these outcomes may be a consequence of genetic factors associated with HLHS, and others may be modifiable through changes to procedures and to the healthcare environment. There is an emerging clinical consensus around the importance of continuous neurodevelopmental surveillance from the earliest years into adulthood.[38][39]
As is true for patients with other types of heart defects involving malformed valves,[40] HLHS patients run a high risk of endocarditis, and must be monitored by a cardiologist for the rest of their lives to check on their heart function.
Heart transplantation may be indicated, typically after Fontan completion.[12] One multi-center study (of patients undergoing the Fontan from 1993-2001) reported a 76% 1-year survival rate in patients who survived to transplant.[41]
Epidemiology
HLHS occurs in an estimated 1 out of 4,300 live births in the United States, or an estimated total of 960 live births per year in that country.[42][43] Overall, it is estimated to make up 2-3% of all cases of congenital heart disease, and is the most common single-ventricle defect. It is thought to be more common in male infants, 1.5 times as common as in female infants.[11]
References
- ↑ "Hypoplastic left heart syndrome: MedlinePlus Medical Encyclopedia". medlineplus.gov. Archived from the original on 28 May 2019. Retrieved 28 May 2019.
- 1 2 3 4 "Congenital Heart Defects - Facts about Hypoplastic Left Heart Syndrome". Centers for Disease Control and Prevention. 19 November 2019. Archived from the original on 1 February 2021. Retrieved 6 February 2021.
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 Kritzmire, SM; Cossu, AE (January 2020). "Hypoplastic Left Heart Syndrome". PMID 32119463.
{{cite journal}}: Cite journal requires|journal=(help) - ↑ Rychik, Jack; Wernovsky, Gil (2003). Hypoplastic Left Heart Syndrome. Springer Science & Business Media. p. 39. ISBN 978-1-4020-7319-9. Archived from the original on 2021-08-28. Retrieved 2021-02-04.
- 1 2 3 4 5 6 7 8 Fulton, David R. (October 26, 2017). "Hypoplastic left heart syndrome". Up To Date. Archived from the original on 2019-12-23. Retrieved 2017-11-30.
- 1 2 Barron, D. J., Kilby, M. D., Davies, B., Wright, J. G., Jones, T. J., & Brawn, W. J. (2009). "Hypoplastic left heart syndrome". The Lancet. 374 (9689): 551–564. doi:10.1016/s0140-6736(09)60563-8. PMID 19683641.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ↑ Hinton, R. B., Martin, L. J., Tabangin, M. E., Mazwi, M. L., Cripe, L. H., & Benson, D. W. (2007). "Hypoplastic left heart syndrome is heritable". Journal of the American College of Cardiology. 50 (16): 1590–1595. doi:10.1016/j.jacc.2007.07.021. PMID 17936159.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ↑ Dasgupta C, Martinez AM, Zuppan CW, Shah MM, Bailey LL, Fletcher WH (2001). "Identification of connexin43 (alpha1) gap junction gene mutations in patients with hypoplastic left heart syndrome by denaturing gradient gel electrophoresis (DGGE)". Mutat. Res. 479 (1–2): 173–86. doi:10.1016/S0027-5107(01)00160-9. PMID 11470490.
- 1 2 Schidlow, David N.; Freud, Lindsay; Friedman, Kevin; Tworetzky, Wayne (2017). "Fetal interventions for structural heart disease". Echocardiography. 34 (12): 1834–1841. doi:10.1111/echo.13667. ISSN 1540-8175. Archived from the original on 2021-04-27. Retrieved 2020-03-09.
- ↑ Eghtesady P, Brar A, Hall M (February 2011). "Seasonality of hypoplastic left heart syndrome in the United States: a 10-year time-series analysis". J. Thorac. Cardiovasc. Surg. 141 (2): 432–8. doi:10.1016/j.jtcvs.2010.06.060. PMID 20817208.
- 1 2 3 4 5 Fulton, David R. (October 26, 2017). "Hypoplastic left heart syndrome". Up To Date. Archived from the original on 2019-12-23. Retrieved 2017-11-30.
- 1 2 3 4 5 6 Feinstein, JA; Benson, DW; Dubin, AM; Cohen, MS; Maxey, DM; Mahle, WT; Pahl, E; Villafañe, J; Bhatt, AB; Peng, LF; Johnson, BA; Marsden, AL; Daniels, CJ; Rudd, NA; Caldarone, CA; Mussatto, KA; Morales, DL; Ivy, DD; Gaynor, JW; Tweddell, JS; Deal, BJ; Furck, AK; Rosenthal, GL; Ohye, RG; Ghanayem, NS; Cheatham, JP; Tworetzky, W; Martin, GR (3 January 2012). "Hypoplastic left heart syndrome: current considerations and expectations". Journal of the American College of Cardiology. 59 (1 Suppl): S1–42. doi:10.1016/j.jacc.2011.09.022. PMC 6110391. PMID 22192720.
- ↑ Hinton, R. B., Martin, L. J., Rame-Gowda, S., Tabangin, M. E., Cripe, L. H., & Benson, D. W. (2009). "Hypoplastic left heart syndrome links to chromosomes 10q and 6q and is genetically related to bicuspid aortic valve". Journal of the American College of Cardiology. 53 (12): 1065–1071. doi:10.1016/j.jacc.2008.12.023. PMC 2703749. PMID 19298921.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - 1 2 Cunningham, F. Gary; Leveno, Kenneth J.; Bloom, Steven L.; Spong, Catherine Y.; Dashe, Jodi S.; Hoffman, Barbara L.; Casey, Brian M.; Sheffield, Jeanne S. (2013). "Fetal Imaging". Williams Obstetrics (24 ed.). New York, NY: McGraw-Hill Education. Archived from the original on 2019-12-23. Retrieved 2017-11-30.
- ↑ Galindo, A., Nieto, O., Villagrá, S., Grañeras, A., Herraiz, I., & Mendoza, A. (2009). "Hypoplastic left heart syndrome diagnosed in fetal life: associated findings, pregnancy outcome and results of palliative surgery". Ultrasound in Obstetrics & Gynecology. 33 (5): 560–566. doi:10.1002/uog.6355. PMID 19367583.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ↑ Burns, Paul Burns and Jasper. "Hypoplastic Left Heart Syndrome | Congenital Heart Disease - Cove Point Foundation | Johns Hopkins Children's Hospital". www.pted.org. Archived from the original on 2017-04-02. Retrieved 2017-11-30.
- ↑ Noseda C, Mialet-Marty T, Basquin A (April 2012). "Hypoplasies sévères du ventricule gauche : soins palliatifs après un diagnostic prénatal". Archives de pediatrie. 19 (4): 374–380. doi:10.1016/j.arcped.2012.01.022. PMID 22397767.
- ↑ Green A, Pye S, Yetman AT (August 2002). "The physiologic basis for and nursing considerations in the use of subatmospheric concentrations of oxygen in HLHS". Advances in Neonatal Care. 2 (4): 177–86. doi:10.1053/adnc.2002.33542. PMID 12881932.
- ↑ Khambadkone S.; Li J.; De Leval M. R.; Cullen S.; Deanfield J. E.; Redington A. N. (2003). "Basal pulmonary vascular resistance and nitric oxide responsiveness late after Fontan-type operation". Circulation. 107 (25): 3204–3208. doi:10.1161/01.cir.0000074210.49434.40. PMID 12821557.
- ↑ Norwood W. I. (1991). "Hypoplastic left heart syndrome. The". Annals of Thoracic Surgery. 52 (3): 688–695. doi:10.1016/0003-4975(91)90978-y. PMID 1898174.
- ↑ "Hypoplastic Left Heart Syndrome (HLHS) | The Children's Hospital of Philadelphia". Archived from the original on 2014-10-14. Retrieved 2013-04-20.
- ↑ McHugh, KE; Hillman, DG; Gurka, MJ; Gutgesell, HP (Jan–Feb 2010). "Three-stage palliation of hypoplastic left heart syndrome in the University HealthSystem Consortium". Congenital Heart Disease. 5 (1): 8–15. doi:10.1111/j.1747-0803.2009.00367.x. PMID 20136852.
- ↑ Hirsch, JC; Gurney, JG; Donohue, JE; Gebremariam, A; Bove, EL; Ohye, RG (July 2008). "Hospital mortality for Norwood and arterial switch operations as a function of institutional volume". Pediatric Cardiology. 29 (4): 713–7. doi:10.1007/s00246-007-9171-2. PMID 18080151.
- 1 2 Vojtovič, P.; Tláskal, T.; Gebauer, R.; Reich, O.; Chaloupecký, V.; Tomek, V.; Krupičková, S.; Matějka, T.; Hecht, P. (December 2014). "Long-term results of children operated for hypoplastic left heart syndrome in Children's Heart Centre". Cor et Vasa. 56 (6): e449–e455. doi:10.1016/j.crvasa.2014.07.006. ISSN 0010-8650.
- ↑ Mussatto, Kathleen A.; Hoffmann, Raymond G.; Hoffman, George M.; Tweddell, James S.; Bear, Laurel; Cao, Yumei; Brosig, Cheryl (2014). "Risk and prevalence of developmental delay in young children with congenital heart disease". Pediatrics. 133 (3): e570–577. doi:10.1542/peds.2013-2309. ISSN 1098-4275. PMC 3934337. PMID 24488746.
- ↑ Wernovsky, Gil; Licht, Daniel J. (2016). "Neurodevelopmental Outcomes in Children with Congenital Heart Disease – What can we impact?". Pediatric Critical Care Medicine. 17 (8 Suppl 1): S232–S242. doi:10.1097/PCC.0000000000000800. ISSN 1529-7535. PMC 4975480. PMID 27490605.
- ↑ Yabrodi, Mouhammad; Mastropietro, Christopher W. (2016-10-04). "Hypoplastic left heart syndrome: from comfort care to long-term survival". Pediatric Research. 81 (1–2): 142–149. doi:10.1038/pr.2016.194. ISSN 0031-3998. PMC 5313512. PMID 27701379.
- ↑ Wernovsky, Gil (1 September 2008). "The Paradigm Shift Toward Surgical Intervention for Neonates With Hypoplastic Left Heart Syndrome". Archives of Pediatrics & Adolescent Medicine. 162 (9): 849–54. doi:10.1001/archpedi.162.9.849. PMID 18762602.
- ↑ Kon, Alexander A.; Ackerson, Lynn; Lo, Bernard (31 May 2003). "Choices physicians would make if they were the parents of a child with hypoplastic left heart syndrome". The American Journal of Cardiology. 91 (12): 1506–1509. doi:10.1016/S0002-9149(03)00412-0. PMID 12804748.
- 1 2 3 4 5 6 7 Feinstein, Jeffrey A.; Benson, D. Woodrow; Dubin, Anne M.; Cohen, Meryl S.; Maxey, Dawn M.; Mahle, William T.; Pahl, Elfriede; Villafañe, Juan; Bhatt, Ami B. (Jan 2012). "Hypoplastic Left Heart Syndrome". Journal of the American College of Cardiology. 59 (1): S1–S42. doi:10.1016/j.jacc.2011.09.022. PMC 6110391. PMID 22192720.
- ↑ "new norwood.gif". Archived from the original on November 24, 2010.
- ↑ Murphy, Michael O.; Bellsham-Revell, Hannah; Morgan, Gareth J.; Krasemann, Thomas; Rosenthal, Eric; Qureshi, Shakeel A.; Salih, Caner; Austin, Conal B.; Anderson, David R. (2015). "Hybrid Procedure for Neonates With Hypoplastic Left Heart Syndrome at High-Risk for Norwood: Midterm Outcomes". The Annals of Thoracic Surgery. 100 (6): 2286–2292. doi:10.1016/j.athoracsur.2015.06.098. PMID 26433522.
- ↑ Chauhan, Monika; Mastropietro, Christopher W. (2014). "Hypoplastic Left Heart Syndrome in the Emergency Department: An Update". The Journal of Emergency Medicine. 46 (2): e51–e54. doi:10.1016/j.jemermed.2013.08.061. PMID 24188609.
- ↑ "Children's Hospital Boston | Pediatric Views". Archived from the original on 2012-02-29. Retrieved 2010-05-14.
- ↑ Galantowicz M, Cheatham JP, Phillips A, et al. (June 2008). "Hybrid approach for hypoplastic left heart syndrome: intermediate results after the learning curve". Ann. Thorac. Surg. 85 (6): 2063–70, discussion 2070–1. doi:10.1016/j.athoracsur.2008.02.009. PMID 18498821.
- ↑ Nieves, Jo Ann; Uzark, Karen; Rudd, Nancy A.; Strawn, Jennifer; Schmelzer, Anne; Dobrolet, Nancy (2017-04-01). "Interstage Home Monitoring After Newborn First-Stage Palliation for Hypoplastic Left Heart Syndrome: Family Education Strategies". Critical Care Nurse. 37 (2): 72–88. doi:10.4037/ccn2017763. ISSN 0279-5442.
- ↑ White, Brian R.; Rogers, Lindsay S.; Kirschen, Matthew P. (2019). "Recent advances in our understanding of neurodevelopmental outcomes in congenital heart disease". Current Opinion in Pediatrics. 31 (6): 783–788. doi:10.1097/MOP.0000000000000829. ISSN 1040-8703. PMC 6852883. PMID 31693588.
- ↑ Marino, Bradley S.; Lipkin, Paul H.; Newburger, Jane W.; Peacock, Georgina; Gerdes, Marsha; Gaynor, J. William; Mussatto, Kathleen A.; Uzark, Karen; Goldberg, Caren S. (2012-08-28). "Neurodevelopmental Outcomes in Children With Congenital Heart Disease: Evaluation and Management: A Scientific Statement From the American Heart Association". Circulation. 126 (9): 1143–1172. doi:10.1161/CIR.0b013e318265ee8a. ISSN 0009-7322. PMID 22851541.
- ↑ Gurvitz, Michelle; Burns, Kristin M.; Brindis, Ralph; Broberg, Craig S.; Daniels, Curt J.; Fuller, Stephanie M.P.N.; Honein, Margaret A.; Khairy, Paul; Kuehl, Karen S. (2016-04-26). "Emerging Research Directions in Adult Congenital Heart Disease: A Report from a National Heart, Lung, and Blood Institute/Adult Congenital Heart Association Working Group". Journal of the American College of Cardiology. 67 (16): 1956–1964. doi:10.1016/j.jacc.2016.01.062. ISSN 0735-1097. PMC 4846980. PMID 27102511.
- ↑ "Endocarditis: Risk factors". MayoClinic.com. Archived from the original on 2007-10-28. Retrieved 2007-10-23.
- ↑ Taylor, D. O., Stehlik, J., Edwards, L. B., Aurora, P., Christie, J. D., Dobbels, F., ... & Hertz, M. I. (2009). "Registry of the International Society for Heart and Lung Transplantation: twenty-sixth official adult heart transplant report—2009". The Journal of Heart and Lung Transplantation. 28 (10): 1007–1022. doi:10.1016/j.healun.2009.08.014. PMID 19782283.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ↑ "Hypoplastic Left Heart Syndrome Facts | Congenital Heart Defects". Centers for Disease Control and Prevention. 2017-10-26. Archived from the original on 2021-02-01. Retrieved 2017-11-30.
- ↑ Parker SE, Mai CT, Canfield MA, Rickard R, Wang Y, Meyer RE, Anderson P, Mason CA, Collins JS, Kirby RS, Correa A; National Birth Defects Prevention Network. (2010). "Updated National Birth Prevalence estimates for selected birth defects in the United States, 2004-2006". Birth Defects Res a Clin Mol Teratol. 88 (12): 1008–16. doi:10.1002/bdra.20735. PMID 20878909.
{{cite journal}}: CS1 maint: multiple names: authors list (link)
External links
| Classification | |
|---|---|
| External resources |
|
- Hypoplastic left heart syndrome Archived 2013-03-14 at the Wayback Machine information for parents.