Persistent truncus arteriosus
| Persistent truncus arteriosus | |
|---|---|
| Other names: Common arterial trunk, trunks arterioles communis, common aorticopulmonary trunk[1] patent truncus arteriosus | |
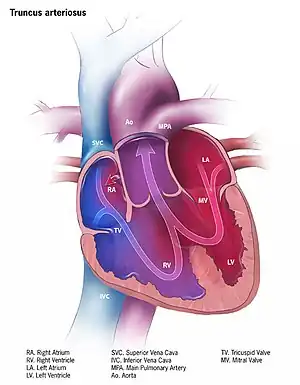 | |
| Illustration of truncus arteriosus | |
| Specialty | Cardiac surgery |
| Symptoms | First few days: Mild bluish skin Later: Poor activity, poor feeding, fast breathing[2] |
| Complications | Heart failure, Eisenmenger syndrome[3] |
| Usual onset | Present at birth[2] |
| Causes | Unknown[3] |
| Risk factors | DiGeorge syndrome[3] |
| Diagnostic method | Ultrasound of the heart[2] |
| Differential diagnosis | Tetralogy of Fallot, tricuspid atresia, total anomalous pulmonary venous return, hypoplastic left heart syndrome, coarctation of the aorta[3] |
| Treatment | Prostaglandin E1, breathing support, furosemide, surgery[4][3] |
| Prognosis | 20 year survival >80% with treatment[3] |
| Frequency | 7 per 100,000 live births[3] |
Persistent truncus arteriosus (PTA) is a congenital heart disease that presents with a common ventricular outflow tract, ventricular septal defect (VSD), and single truncal valve.[2] In the first few days of life there may be no symptoms, with the development of bluish skin over the next few weeks.[2] Without surgery, this is followed by heart failure with poor activity, poor feeding, and fast breathing.[3] Complications may include Eisenmenger syndrome.[3]
The cause is generally unknown, though the condition may be associated with DiGeorge syndrome.[3] The underlying mechanism involves failure of the truncus arteriosus to divide into the pulmonary trunk and aorta during early development.[3] This results in blood from both sides of the heart mixing before it enters the coronary arteries, pulmonary arteries, and systemic circulation.[4][3] One in four cases have significant truncal valve narrowing and back flow of blood.[2] The condition may be suspected based on an ECG showing enlargement of both ventricles, a chest X-ray showing a large heart, and a systolic heart murmur.[4][3] Confirmation is by ultrasound of the heart.[3]
Initial treatment may include prostaglandin E1 and breathing support.[4] If pulmonary edema is present furosemide, CPAP, and avoiding extra oxygen may be recommended.[3] Surgery correction is recommended within the first month of life.[3] About 75% of people require a further operation in their next 10 years of life.[3] Without treatment, around 40% do not survive beyond one month.[2] Around 5% do not survive surgery, but of those that do, survival is 90% at 5 years, 85% at 10-years, and 83% at 15 years.[2]
PTA is rare, affecting about 7 per 100,000 live births.[3] It accounts for around 0.4% to 4% of all heart defects that present at birth.[2] The work "truncus arteriosus" is Latin for single artery.[3] It was first described by James Wilson in 1798.[1]
Signs and symptoms
In the first few days of life bluish skin is generally mild.[3] Without treatment, this is followed by heart failure with poor activity, poor feeding, and fast breathing.[3] Complications may include Eisenmenger syndrome.[3]
Causes
Most of the time, this defect occurs spontaneously. Genetic disorders, and teratogens (viruses, metabolic imbalance, and industrial or pharmacological agents) have been associated as possible causes. Up to 50% (varies in studies) of cases are associated with chromosome 22q11 deletions (DiGeorge syndrome). The neural crest, specifically a population known as the cardiac neural crest, directly contributes to the aorticopulmonary septum.[5][6]
Microablation of the cardiac neural crest in developing chick embryos and genetic anomalies affecting this population of cells in rodents results in persistent truncus arteriosus.[7][8][9]
Numerous perturbations affecting the cardiac neural crest have been associated with persistent truncus arteriosus, some of which include growth factors (fibroblast growth factor 8 and bone morphogenetic protein), transcription factors (T-box, Pax, Nkx2-5, GATA-6, and Forkhead), and gap junction proteins (Connexin). The cardiac neural crest also contributes the smooth muscle of the great arteries.
Pathophysiology
Anatomical changes associated with this disorder includes:
- single artery arising from the two ventricles which gives rise to both the aortic and pulmonary vessels
- abnormal truncal valve
- right sided aortic arch in about 30% of cases (not shown)
- large ventricular septal defect
- pulmonary hypertension
- complete mixing occurring at level of the great vessel
- right-to-left shunting of blood
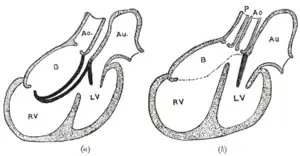 Diagrams to illustrate the transformation of the bulbus cordis. Ao. Truncus arteriosus. Au. Atrium. B. Bulbus cordis. RV. Right ventricle. LV. Left ventricle. P. Pulmonary artery.
Diagrams to illustrate the transformation of the bulbus cordis. Ao. Truncus arteriosus. Au. Atrium. B. Bulbus cordis. RV. Right ventricle. LV. Left ventricle. P. Pulmonary artery.
Diagnosis
- Cyanosis presents at birth
- Heart failure may occur within weeks
- Systolic ejection murmur is heard at the left sternal border
- Widened pulse pressure
- Bounding arterial pulses
- Loud second heart sound
- Biventricular hypertrophy
- Cardiomegaly
- Increased pulmonary vascularity
- Hypocalcemia (if associated with DiGeorge syndrome)
Classification
The most well-known classification was the fourfold system developed by Collett and Edwards in 1949.[10] Collett/Edwards Types I, II, and III are distinguished by the branching pattern of the pulmonary arteries:[11][12]
- Type I: truncus -> one pulmonary artery -> two lateral pulmonary arteries
- Type II: truncus -> two posterior/posterolateral pulmonary arteries
- Type III: truncus -> two lateral pulmonary arteries
The "Type IV" proposed in 1949 is no longer considered a form of PTA by most modern sources.[12]
Another well-known classification was defined by Van Praaghs in 1965.[12][13]
Treatment
Treatment is with neonatal surgical repair, with the objective of restoring a normal pattern of blood flow.[14] The surgery is open heart, and the patient will be placed on cardiopulmonary bypass to allow the surgeon to work on a still heart. The heart is opened and the ventricular septal defect is closed with a patch. The pulmonary arteries are then detached from the common artery (truncus arteriosus) and connected to the right ventricle using a tube (a conduit or tunnel). The common artery, now separated from the pulmonary circulation, functions as the aorta with the truncal valve operating as the aortic valve. Most babies survive this surgical repair, but may require further surgery as they grow up. For example, the conduit does not grow with the child and may need to be replaced as the child grows. Furthermore, the truncal valve is often abnormal and may require future surgery to improve its function. There have been cases where the condition has been diagnosed at birth and surgical intervention is an option. A number of these cases have survived well into adulthood.[15]
Epidemiology
Persistent truncus arteriosus is a rare cardiac abnormality that has a prevalence of less than 1%.[4][16]
History
It was first described by James Wilson in 1798.[1] Since Lev and Saphir's first anatomic criteria to define the disease in 1942, several varying classifications have been proposed including one by Collett and Edwards in 1942, one by Van Pragh in 1965, and one in 2000 classification by the Society of Thoracic Surgeons.[1]
References
- 1 2 3 4 Chan, Francis P. (2011). "45. Truncus arteriosus". In Ho, Vincent B.; Reddy, Gautham P. (eds.). Cardiovascular Imaging. Vol. 1. St. Louis, Missouri: Saunders, Elsevier. pp. 616–624. ISBN 978-1-4160-5335-4. Archived from the original on 2022-10-13. Retrieved 2021-02-04.
- 1 2 3 4 5 6 7 8 9 Sainathan, Sandeep W.; Bayle, Ken-Michael; Knott-Craig, Christopher; Boston, Umar (2020). "98. Truncus arteriosus". In Raja, Shahzad G. (ed.). Cardiac Surgery: A Complete Guide. Switzerland: Springer. pp. 891–895. ISBN 978-3-030-24176-6. Archived from the original on 2022-10-16. Retrieved 2022-10-16.
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 Bhansali, S; Phoon, C (January 2020). "Truncus Arteriosus". PMID 30521195.
{{cite journal}}: Cite journal requires|journal=(help) - 1 2 3 4 5 Barata IA (August 2013). "Cardiac emergencies". Emergency Medicine Clinics of North America. 31 (3): 677–704. doi:10.1016/j.emc.2013.04.007. PMID 23915599.
- ↑ Kirby ML, Gale TF, Stewart DE (June 1983). "Neural crest cells contribute to normal aorticopulmonary septation". Science. 220 (4601): 1059–61. Bibcode:1983Sci...220.1059K. doi:10.1126/science.6844926. PMID 6844926.
- ↑ Jiang X, Rowitch DH, Soriano P, McMahon AP, Sucov HM (April 2000). "Fate of the mammalian cardiac neural crest". Development. 127 (8): 1607–16. PMID 10725237.
- ↑ Hutson MR, Kirby ML (February 2003). "Neural crest and cardiovascular development: a 20-year perspective". Birth Defects Research. Part C, Embryo Today. 69 (1): 2–13. doi:10.1002/bdrc.10002. PMID 12768653.
- ↑ Waller BR, McQuinn T, Phelps AL, Markwald RR, Lo CW, Thompson RP, Wessels A (November 2000). "Conotruncal anomalies in the trisomy 16 mouse: an immunohistochemical analysis with emphasis on the involvement of the neural crest". The Anatomical Record. 260 (3): 279–93. doi:10.1002/1097-0185(20001101)260:3<279::AID-AR65>3.0.CO;2-2. PMID 11066038.
- ↑ Franz T (1989). "Persistent truncus arteriosus in the Splotch mutant mouse". Anatomy and Embryology. 180 (5): 457–64. doi:10.1007/BF00305120. PMID 2619088. S2CID 24116967.
- ↑ Collett RW, Edwards JE: Persistent truncus arteriosus: a classification according to anatomic types. Surg Clin North Am 1949; 29: 1245-70.
- ↑ "Persistent Truncus Arteriosus: Congenital Cardiovascular Anomalies: Merck Manual Professional". Archived from the original on 2010-11-04. Retrieved 2007-11-04.
- 1 2 3 "eMedicine - Truncus Arteriosus : Article by Doff McElhinney, MD". Archived from the original on 2008-12-07. Retrieved 2007-11-04.
- ↑ Van Praagh R, Van Praagh S (September 1965). "The anatomy of common aorticopulmonary trunk (truncus arteriosus communis) and its embryologic implications. A study of 57 necropsy cases". The American Journal of Cardiology. 16 (3): 406–25. doi:10.1016/0002-9149(65)90732-0. PMID 5828135.
- ↑ Rodefeld MD, Hanley FL (2002). "Neonatal truncus arteriosus repair: surgical techniques and clinical management". Seminars in Thoracic and Cardiovascular Surgery. Pediatric Cardiac Surgery Annual. 5 (1): 212–7. doi:10.1053/pcsu.2002.31497. PMID 11994881.
- ↑ Haskal ZJ (2005). "SIR 2005 Annual Meeting Film Panel Case: hemoptysis and bronchial artery embolization in an adult with uncorrected truncus arteriosus and Eisenmenger syndrome". Journal of Vascular and Interventional Radiology. 16 (5): 635–8. doi:10.1097/01.RVI.0000161372.87971.84. PMID 15872317.
- ↑ Parker SE, Mai CT, Canfield MA, Rickard R, Wang Y, Meyer RE, Anderson P, Mason CA, Collins JS, Kirby RS, Correa A (December 2010). "Updated National Birth Prevalence estimates for selected birth defects in the United States, 2004-2006". Birth Defects Research. Part A, Clinical and Molecular Teratology. 88 (12): 1008–16. doi:10.1002/bdra.20735. PMID 20878909.
External links
| Classification | |
|---|---|
| External resources |