Deferoxamine
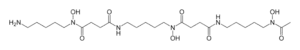 | |
 Skeletal formula and spacefill model of deferoxamine | |
| Names | |
|---|---|
| Trade names | Desferal |
| Other names | desferrioxamine B, desferoxamine B, DFO-B, DFB ,N'-[5-(Acetyl-hydroxy-amino)pentyl]-N-[5-[3-(5-aminopentyl-hydroxy-carbamoyl) propanoylamino]pentyl]-N-hydroxy-butane diamide |
IUPAC name
| |
| Clinical data | |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Pregnancy category |
|
| Routes of use |
|
| Defined daily dose | not established[1] |
| External links | |
| AHFS/Drugs.com | Monograph |
| Pharmacokinetics | |
| Elimination half-life | 6 hours |
| Identifiers | |
| CAS Number |
|
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| ATC code | |
| Chemical and physical data | |
| Formula | C25H48N6O8 |
| Molar mass | 560.693 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Deferoxamine (DFOA), sold under the brand name Desferal, is a medication that binds iron and aluminium.[2] It is specifically used in iron overdose, hemochromatosis either due to multiple blood transfusions or an underlying genetic condition, and aluminium toxicity in people on dialysis.[2][3] It is used by injection into a muscle, vein, or under the skin.[2]
Common side effects include pain at the site of injection, diarrhea, vomiting, fever, hearing loss, and eye problems.[2] Severe allergic reactions including anaphylaxis and low blood pressure may occur.[2] It is unclear if use during pregnancy or breastfeeding is safe for the baby.[4] Deferoxamine is a siderophore from the bacteria Streptomyces pilosus.[5][6]
Deferoxamine was approved for medical use in the United States in 1968.[2] It is on the World Health Organization's List of Essential Medicines.[7] The wholesale cost in the developing world is about US$6.76 to $13.52 per dose.[8] In the United States a course of treatment costs more than $200.[9]
Medical uses
Deferoxamine is used to treat acute iron poisoning, especially in small children.[10] This agent is also frequently used to treat hemochromatosis, a disease of iron accumulation that can be either genetic or acquired. Acquired hemochromatosis is common in patients with certain types of chronic anemia (e.g. thalassemia and myelodysplastic syndrome) who require many blood transfusions, which can greatly increase the amount of iron in the body. Treatment with iron-chelating drugs such as deferoxamine reduces mortality in persons with sickle cell disease or β‐thalassemia who are transfusion dependent.[11]
Administration for chronic conditions is generally accomplished by subcutaneous injection over a period of 8–12 hours each day. Administration of deferoxamine after acute intoxication may color the urine a pinkish red, a phenomenon termed "vin rosé urine". Apart from iron toxicity, deferoxamine can be used to treat aluminium toxicity (an excess of aluminium in the body) in select patients. In US, the drug is not FDA-approved for this use. Deferoxamine is also used to minimize doxorubicin's cardiotoxic side effects and in the treatment of a patient with aceruloplasminemia.[12] Deferoxamine maybe effective for improving neurologic outcomes in persons with intracranial hemorrhage, although the evidence supporting the efficacy and safety for this indication was weak.[13]
Dosage
The defined daily dose is not established[1]
Side effects
It is unclear if use during pregnancy is safe for the baby.[4]
Chronic use of deferoxamine may increase the risk of hearing loss in patients with thalassemia major.[14]
Chronic use of deferoxamine may increase the risk of visual loss.
Mechanism
Deferoxamine is produced by removal of the trivalent iron moiety from ferrioxamine B, an iron-bearing sideramine produced by the actinomycetes, Streptomyces pilosus. Its discovery was a serendipitous result of research conducted by scientists at Ciba in collaboration with scientists at the Swiss Federal Institute of Technology in Zurich and the University Hospital in Freiburg, Germany[15][5] Deferoxamine acts by binding free iron in the bloodstream and enhancing its elimination in the urine. By removing excess iron from persons with hemochromatosis, the agent reduces the damage done to various organs and tissues, such as the liver. Also, it speeds healing of nerve damage (and minimizes the extent of recent nerve trauma). Deferoxamine may modulate expression[16] and release of inflammatory mediators by specific cell types.[17]
Research
Deferoxamine is being studied as a treatment for spinal cord injury[18] and intracerebral hemorrhage.[19][20] It is also used to induce hypoxia-like environment in mesenchymal stem cells.[21][22]
Costs
In the United States, a course of treatment has been estimated to have a retail cost, before insurance or discounts, of more than $200.[9]
See also
References
- 1 2 "WHOCC - ATC/DDD Index". www.whocc.no. Archived from the original on 23 January 2021. Retrieved 20 September 2020.
- 1 2 3 4 5 6 "Deferoxamine Mesylate". The American Society of Health-System Pharmacists. Archived from the original on 21 December 2016. Retrieved 8 December 2016.
- ↑ World Health Organization (2009). Stuart MC, Kouimtzi M, Hill SR (eds.). WHO Model Formulary 2008. World Health Organization. pp. 61–62. hdl:10665/44053. ISBN 9789241547659.
- 1 2 "Deferoxamine (Desferal) Use During Pregnancy". www.drugs.com. Archived from the original on 21 December 2016. Retrieved 13 December 2016.
- 1 2 Hoffman, Ronald; Jr, Edward J. Benz; Silberstein, Leslie E.; Heslop, Helen; Weitz, Jeffrey; Anastasi, John (2012). Hematology: Diagnosis and Treatment (6 ed.). Elsevier Health Sciences. p. 515. ISBN 978-1-4557-4041-3. Archived from the original on 2016-12-20.
- ↑ KEBERLE, H (7 October 1964). "THE BIOCHEMISTRY OF DESFERRIOXAMINE AND ITS RELATION TO IRON METABOLISM". Annals of the New York Academy of Sciences. 119: 758–68. doi:10.1111/j.1749-6632.1965.tb54077.x. PMID 14219455.
- ↑ World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- ↑ "Deferoxamine". International Drug Price Indicator Guide. Archived from the original on 22 January 2018. Retrieved 8 December 2016.
- 1 2 Hamilton, Richart (2015). Tarascon Pocket Pharmacopoeia 2015 Deluxe Lab-Coat Edition. Jones & Bartlett Learning. p. 470. ISBN 978-1-284-05756-0.
- ↑ Merlot, Angelica M.; Kalinowski, Danuta S.; Richardson, Des R. (2013). "Novel Chelators for Cancer Treatment: Where Are We Now?". Antioxidants & Redox Signaling. 18 (8): 973–1006. doi:10.1089/ars.2012.4540. PMID 22424293.
- ↑ Ballas SK, Zeidan AM, Duong VH, DeVeaux M, Heeney MM (July 2018). "The effect of iron chelation therapy on overall survival in sickle cell disease and β-thalassemia: A systematic review". Am. J. Hematol. 93 (7): 943–952. doi:10.1002/ajh.25103. PMID 29635754.
- ↑ Miyajima, Hiroaki; Takahashi, Yoshitomo; Kamata, Tadashi; Shimizu, Hideaki; Sakai, Naoki; Gitlin, Jonathan D. (1997). "Use of desferrioxamine in the treatment of aceruloplasminemia". Annals of Neurology. 41 (3): 404–7. doi:10.1002/ana.410410318. PMID 9066364.
- ↑ Zeng L, Tan L, Li H, Zhang Q, Li Y, Guo J (2018). "Deferoxamine therapy for intracerebral hemorrhage: A systematic review". PLoS ONE. 13 (3): e0193615. Bibcode:2018PLoSO..1393615Z. doi:10.1371/journal.pone.0193615. PMC 5863956. PMID 29566000.
- ↑ Badfar G, Mansouri A, Shohani M, Karimi H, Khalighi Z, Rahmati S, Delpisheh A, Veisani Y, Soleymani A, Azami M (2017). "Hearing loss in Iranian thalassemia major patients treated with deferoxamine: A systematic review and meta-analysis". Caspian J Intern Med. 8 (4): 239–249. doi:10.22088/cjim.8.4.239. PMC 5686301. PMID 29201313.
- ↑ Yawalkar SJ (1993). "Milestones in the research and development of desferrioxamine". Nephrol. Dial. Transplant. 8 Suppl 1: 40–2. doi:10.1093/ndt/8.supp1.40. PMID 8389019.
- ↑ Lee, Hwa-Jeong; Lee, Jun; Lee, Sun-Kyung; Lee, Suk-Keun; Kim, Eun-Cheol (2007). "Differential regulation of iron chelator-induced IL-8 synthesis via MAP kinase and NF-κB in immortalized and malignant oral keratinocytes". BMC Cancer. 7: 176. doi:10.1186/1471-2407-7-176. PMC 2078595. PMID 17850672.
- ↑ Choi, E. Y.; Kim, E. C.; Oh, H. M.; Kim, S; Lee, H. J.; Cho, E. Y.; Yoon, K. H.; Kim, E. A.; Han, W. C.; Choi, S. C.; Hwang, J. Y.; Park, C; Oh, B. S.; Kim, Y; Kimm, K. C.; Park, K. I.; Chung, H. T.; Jun, C. D. (2004). "Iron chelator triggers inflammatory signals in human intestinal epithelial cells: Involvement of p38 and extracellular signal-regulated kinase signaling pathways". Journal of Immunology. 172 (11): 7069–77. doi:10.4049/jimmunol.172.11.7069. PMID 15153529.
- ↑ http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/orphans/2009/11/human_orphan_000120.jsp&mid=WC0b01ac058001d12b Archived 2013-07-17 at the Wayback Machine
- ↑ Wu, He; Wu, Tao; Xu, Xueying; Wang, Jessica; Wang, Jian (2010). "Iron toxicity in mice with collagenase-induced intracerebral hemorrhage". Journal of Cerebral Blood Flow & Metabolism. 31 (5): 1243–50. doi:10.1038/jcbfm.2010.209. PMC 3099628. PMID 21102602.
- ↑ Ren H, Han R, Chen X, Liu X, Wan J, Wang L, Yang X, Wang J (May 2020). "Potential therapeutic targets for intracerebral hemorrhage-associated inflammation: An update". J Cereb Blood Flow Metab. doi:10.1177/0271678X20923551. PMID 32423330.
- ↑ Ren, Hongying; Cao, Ying; Zhao, Qinjun; Li, Jing; Zhou, Cixiang; Liao, Lianming; Jia, Mingyue; Zhao, Qian; Cai, Huiguo; Han, Zhong Chao; Yang, Renchi; Chen, Guoqiang; Zhao, Robert Chunhua (2006). "Proliferation and differentiation of bone marrow stromal cells under hypoxic conditions". Biochemical and Biophysical Research Communications. 347 (1): 12–21. doi:10.1016/j.bbrc.2006.05.169. PMID 16814746.
- ↑ Woo, Kyung Jin; Lee, Tae-Jin; Park, Jong-Wook; Kwon, Taeg Kyu (2006). "Desferrioxamine, an iron chelator, enhances HIF-1α accumulation via cyclooxygenase-2 signaling pathway". Biochemical and Biophysical Research Communications. 343 (1): 8–14. doi:10.1016/j.bbrc.2006.02.116. PMID 16527254.