Flortaucipir (18F)
 | |
| Names | |
|---|---|
| Pronunciation | flor tau' si pir 18 F |
| Trade names | Tauvid |
| Other names | 18F-AV-1451, 18F-AV-1451, 18F-T807, Flortaucipir F-18, flortaucipir F 18 (USAN US) |
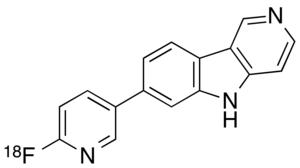
IUPAC name
| |
| Clinical data | |
| Drug class | Radioactive diagnostic agent[1] |
| Main uses | Positron emission tomography (PET) imaging in Alzheimer[1] |
| Side effects | Headache, injection site pain, increased blood pressure[1] |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Routes of use | Intravenous |
| Legal | |
| License data |
|
| Legal status | |
| Chemical and physical data | |
| Formula | C16H10[18F]N3 |
| Molar mass | 262.27 g/mol |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Flortaucipir (18F), sold under the brand name Tauvid, is a radioactive diagnostic agent used in positron emission tomography (PET) imaging of the brain in Alzheimer.[1] It is not useful for chronic traumatic encephalopathy (CTE).[1] It is given by injection into a vein.[1]
Common side effects include headache, injection site pain, and increased blood pressure.[1] Other side effects include radiation exposure.[1] It works by binding to sites of the brain associated with tau protein misfolding.[2]
Flortaucipir (18F) was approved for medical use in the United States in 2020.[1] It is not approved for use in Europe or the United Kingdom as of 2022.[3]
Medical uses
Flortaucipir (18F) is a radioactive diagnostic agent for adults with cognitive impairment who are being evaluated for Alzheimer's disease.[1] It is indicated for positron emission tomography (PET) imaging of the brain to estimate the density and distribution of aggregated tau neurofibrillary tangles (NFTs), a primary marker of Alzheimer's disease.[2][1][4]
Flortaucipir (18F) is not indicated for use in the evaluation of people for chronic traumatic encephalopathy (CTE).[2][1]
Dosage
It is given at a dose of 370 MBq (10 mCi), with imaging following 80 minutes later.[1]
Mechanism of action
Two proteins – tau and amyloid – are recognized as hallmarks of Alzheimer's disease.[2] In people with Alzheimer's disease, pathological forms of tau proteins develop inside neurons in the brain, creating neurofibrillary tangles.[2] After flortaucipir (18F) is administered intravenously, it binds to sites in the brain associated with this tau protein misfolding.[2] The brain can then be imaged with a PET scan to help identify the presence of tau pathology.[2][1]
Chemistry
Chemically, flortaucipir F 18 is 7-(6-[F-18]fluoropyridin-3-yl)-5H-pyrido[4,3 b]indole.[1]
History
Flortaucipir (18F) was approved for medical use in the United States in May 2020.[2][5][6] It is the first drug used to help image a distinctive characteristic of Alzheimer's disease in the brain called tau pathology.[2] The U.S. Food and Drug Administration (FDA) considers it to be a first-in-class medication.[7]
The safety and effectiveness of flortaucipir (18F) imaging was evaluated in two clinical studies.[2] In each study, five evaluators read and interpreted the flortaucipir (18F) imaging.[2] The evaluators were blinded to clinical information and interpreted the imaging as positive or negative.[2]
The first study enrolled 156 participants who were terminally ill and agreed to undergo flortaucipir (18F) imaging and participate in a post-mortem brain donation program.[2] In 64 of the participants who died within nine months of the flortaucipir (18F) brain scan, evaluators' reading of the flortaucipir (18F) scan was compared to post-mortem readings from independent pathologists who evaluated the density and distribution of neurofibrillary tangles (NFTs) in the same brain.[2] The study showed evaluators reading the flortaucipir (18F) images had a high probability of correctly evaluating participants with tau pathology and had an average-to-high probability of correctly evaluating participants without tau pathology.[2]
The second study included the same participants with terminal illness as the first study, plus 18 additional participants with terminal illness, and 159 participants with cognitive impairment being evaluated for Alzheimer's disease (the indicated patient population).[2] The study gauged how well flortaucipir (18F) evaluators' readings agreed with each other's assessments of the readings.[2] Perfect reader agreement would be 1, while no reader agreement would be 0.[2] In this study, reader agreement was 0.87 across all 241 participants.[2] In a separate subgroup analysis that included the 82 terminally ill participants diagnosed after death and the 159 participants with cognitive impairment, reader agreement was 0.90 for the participants in the indicated population and 0.82 in the terminally ill participants.[2]
The FDA approved flortaucipir (18F) based on evidence of 1921 participants from 19 trials conducted at 322 sites in the United States, Australia, Belgium, Canada, France, Japan, Netherlands and Poland.[4]
The ability of flortaucipir (18F) to detect tau pathology was assessed in participants with generally severe stages of dementia and may be lower in participants in earlier stages of cognitive decline than in the participants with terminal illness who were studied.[2]
The U.S. Food and Drug Administration (FDA) granted the application for flortaucipir (18F) priority review and it granted approval of Tauvid to Avid Radiopharmaceuticals, Inc.[2]
Names
Flortaucipir (18F) is the international nonproprietary name (INN).[8]
References
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 "Tauvid- flortaucipir f-18 injection, solution". DailyMed. 22 July 2020. Archived from the original on 28 May 2022. Retrieved 28 May 2022.
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 "FDA Approves First Drug to Image Tau Pathology in Patients Being Evaluated for Alzheimer's Disease". U.S. Food and Drug Administration (FDA) (Press release). 28 May 2020. Archived from the original on 4 February 2021. Retrieved 28 May 2020.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - ↑ "Flortaucipir F18". SPS - Specialist Pharmacy Service. 28 November 2020. Archived from the original on 30 January 2022. Retrieved 4 November 2022.
- 1 2 "Drug Trial Snapshot: Tauvid". U.S. Food and Drug Administration (FDA). 28 May 2020. Archived from the original on 18 September 2020. Retrieved 10 June 2020.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - ↑ "Lilly Receives U.S. FDA Approval of Tauvid (flortaucipir F 18 injection) for Use in Patients Being Evaluated for Alzheimer's Disease" (Press release). Eli Lilly. 28 May 2020. Archived from the original on 7 June 2020. Retrieved 28 May 2020 – via PR Newswire.
- ↑ "Drug Approval Package: Tauvid". U.S. Food and Drug Administration (FDA). 16 July 2020. Archived from the original on 17 September 2021. Retrieved 28 May 2022.
- ↑ "New Drug Therapy Approvals 2020". U.S. Food and Drug Administration (FDA). 31 December 2020. Archived from the original on 18 January 2021. Retrieved 17 January 2021.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - ↑ World Health Organization (2016). "International nonproprietary names for pharmaceutical substances (INN): recommended INN: list 76". WHO Drug Information. 30 (3). hdl:10665/331020.
Further reading
- Pontecorvo MJ, Devous MD, Kennedy I, et al. (June 2019). "A multicentre longitudinal study of flortaucipir (18F) in normal ageing, mild cognitive impairment and Alzheimer's disease dementia". Brain. 142 (6): 1723–1735. doi:10.1093/brain/awz090. PMC 6536847. PMID 31009046.
- Schonhaut DR, McMillan CT, Spina S, et al. (October 2017). "18 F-flortaucipir tau positron emission tomography distinguishes established progressive supranuclear palsy from controls and Parkinson disease: A multicenter study". Ann. Neurol. 82 (4): 622–634. doi:10.1002/ana.25060. PMC 5665658. PMID 28980714.
External links
| Identifiers: |
|---|
- "Flortaucipir F18". Drug Information Portal. U.S. National Library of Medicine. Archived from the original on 1 November 2022. Retrieved 20 October 2022.
- Clinical trial number NCT03507257 for "Longitudinal Early-onset Alzheimer's Disease Study Protocol (LEADS)" at ClinicalTrials.gov
- Clinical trial number NCT02278367 for "Clinical Evaluation of 18F-AV-1451" at ClinicalTrials.gov
- Clinical trial number NCT02516046 for "18F-AV-1451 Autopsy Study" at ClinicalTrials.gov
- Clinical trial number NCT03901092 for "A Reader Study to Assess Accuracy and Reliability of Flortaucipir F 18 PET Scan Interpretation" at ClinicalTrials.gov