Flotufolastat F-18
 Flotufolastat F-18 gallium | |
| Names | |
|---|---|
| Trade names | Posluma |
| Other names | 18F-rhPSMA-7.3 |
| Clinical data | |
| Drug class | Radioactive diagnostic agent |
| Main uses | PET imaging of prostate cancer[1] |
| Side effects | Diarrhea, high blood pressure, pain at site of injection[1] |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Routes of use | Intravenous |
| Legal | |
| License data | |
| Legal status | |
| Chemical and physical data | |
| 3D model (JSmol) | |
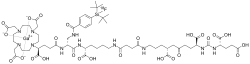
SMILES
| |
InChI
| |
Flotufolastat F-18, sold under the brand name Posluma, is a radioactive diagnostic agent used in positron emission tomography (PET) imaging of prostate cancer.[1] Specifically it is used in prostate-specific membrane antigen (PSMA) positive disease to look for spread or recurrence.[1] It is given by injection into a vein.[1]
Common side effects include diarrhea, high blood pressure, and pain at the site of injection.[1] Other side effects include exposure to radiation.[1] The active ingredient is flotufolastat F-18 gallium.[1]
Flotufolastat F-18 was approved for medical use in the United States in 2023.[1]
Medical uses
Flotufolastat F-18 is indicated for positron emission tomography of prostate-specific membrane antigen positive lesions in men with prostate cancer.[1]
References
External links
| Identifiers: |
|---|
- Clinical trial number NCT04186819 for "Imaging Study to Investigate the Safety and Diagnostic Performance of rhPSMA 7.3 (18F) in Newly Diagnosed Prostate Cancer (LIGHTHOUSE)" at ClinicalTrials.gov
- Clinical trial number NCT04186845 for "Imaging Study to Investigate Safety and Diagnostic Performance of rhPSMA 7.3 (18F) PET Ligand in Suspected Prostate Cancer Recurrence (SPOTLIGHT)" at ClinicalTrials.gov