Gibbon ape leukemia virus
| Gibbon-ape Leukaemia Virus | |
|---|---|
| Virus classification | |
| (unranked): | Virus |
| Realm: | Riboviria |
| Kingdom: | Pararnavirae |
| Phylum: | Artverviricota |
| Class: | Revtraviricetes |
| Order: | Ortervirales |
| Family: | Retroviridae |
| Genus: | Gammaretrovirus |
| Species: | Gibbon-ape Leukaemia Virus |
| Synonyms | |
| |
Gibbon-ape leukemia virus (GaLV) is an oncogenic, type C retrovirus that has been isolated from primate neoplasms, including the white-handed gibbon and woolly monkey.[1] The virus was identified as the etiological agent of hematopoietic neoplasms, leukemias, and immune deficiencies within gibbons in 1971, during the epidemic of the late 1960s and early 1970s. Epidemiological research into the origins of GaLV has developed two hypotheses for the virus' emergence. These include cross-species transmission of the retrovirus present within a species of East Asian rodent or bat, and the inoculation or blood transfusion of a MbRV-related virus into captured gibbons populations housed at medical research institutions.[2] The virus was subsequently identified in captive gibbon populations in Thailand, the US and Bermuda.[3]
GaLV is transmitted horizontally by contact with excretory products of infected gibbons.[4] However, it is also hypothesised to be vertically transmitted via parent-progeny transmission.[5] Phylogenetic analysis have revealed 7 strains of GaLV; GaLV-SF, GaLV-SEATO, GaLV-BR, GALV-X, GaLV-Mar, GaLV-H and SSV, which have emerged as a result of selection pressures from the host immune system.[3] Recently, full genome sequences of these strains have been made available which broadens the possibilities for GaLV's utility as a viral vector in gene transfer.[6]
Epizootiology
History
Cases of malignant lymphomas and leukemias were not described in gibbons until the 1960s, when several cases of haematopoietic neoplasia were reported in a single colony of white-handed gibbons housed at the SEATO research facility in Bangkok, Thailand.[7] In 1971, phylogenetic analysis of the Leukemia-inducing retrovirus, lead to the identification of GaLV-SEATO, published within De Paoli et al. (1971).[3] Following this discovery, five other strains of GaLV was identified from animals whose associated neoplastic syndromes were exclusively recorded in captive gibbon populations, which include:
- GaLV-SF: identified from a gibbon lymphosarcoma in San Francisco and within captured gibbons populations at the University of California San Francisco Medical Center and the University of California. (Kawakami et al. and Snyder et al., 1973)[4]
- GALV‐X: detected in cell culture from a human T-cell line infected with HIV-1 in Louvain, Belgium and at the National Cancer Institute in Maryland, US.[8]
- GALV‐H: identified from a gibbon with lymphocytic leukemias from a colony of free-ranging gibbons at the Hall's Island, Bermuda.[9]
- GALV‐Br: Identified in frozen brain samples of non-leukemic gibbons at the Gulf South Research Institute in Louisiana. (Gallo et al., 1978)[8]
- GaLV-Mar: detected in cell culture (in vitro) derived from marmoset cells.[10]
- Simian sarcoma (SSV): a defective GALV recombinant, derived from a single isolate of fibrosarcoma in a Woolly Monkey that was exposed to a GALV-infected captive gibbon.[2] For viral replication to occur within the host, simian sarcoma-associated virus (SSAV) must also be present.[4]
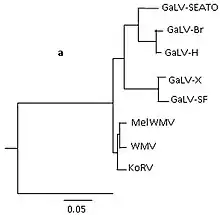
These strains exhibit high genetic similarity, demonstrated through DNA sequencing which reveals approx. 90% sequence identity and more than 93% amino acid genome identity between strains of GaLV. Differences between these strains occurs in the env gene, with divergence ranging from 85% to 99%.[4]
Origins
The discovery of a contagious oncogenic gammaretrovirus in sub-human primates stimulated a great deal of research into the pathogenesis of GaLV and its origins including the virus' intermediate host, which is currently disputed.[2] Virologist initially suggested that GaLV was related to murine leukaemia virus (MLV) detected in Southeast Asian rodents. The endogenous retroviruses with similar homology are; McERV, detected within Mus caroli, and Mus dunni endogenous virus (MDEV) isolated from the earth-coloured mouse (Lieber et al. 1975, Callahan et al. 1979). Furthermore, this hypothesis was based on results derived from low resolution serological and DNA homology methods.[3] Thus, present phylogenetic analysis of proviral sequences of GALV‐SEATO and MLV shows a 68–69% similarity for pol and 55% similarity for env, thus indicating the limited sequence similarity.[3] Therefore, there are no published proviral sequences from rodent hosts which share a sufficiently high degree of sequence identity to GALV to confirm an intermediate rodent host as the precursor for GaLV.[2]
An alternative hypothesis is based on the high sequence similarity of GaLV-SEATO and the Melomys Burtoni retrovirus (MbRV), isolated from a species of rodent from Papua New Guinea. Immunological analysis highlights that MbRV shares 93% sequence homology with GaLV-SEATO which is significantly higher than McERV and MDEV.[2] However, due to the lack of geographic overlap of grassland melomys in PNG and Thailand, MbRV was initially considered ill-suited as the intermediate host of GaLV.[11] However, in 2016 the Mammal Review published "Is gibbon ape leukaemia virus still a threat?" which offered a valid hypothesis for the spread of MbRV from PNG to Thailand by divulging SEATO facility reports and reviewing geographical movement of gibbons during the 1960s and 1970's.[3] The SEATO facility report demonstrated that gibbons were frequently inoculated with biomaterial from humans, Southeast Asian rodents and other gibbons, for pathogenetic study of human diseases including malaria and dengue fever. It is therefore proposed that blood and tissue samples used at SEATO were contaminated with MbRV-related virus and later introduced into Gibbon test subjects via blood transfusion or inoculation, thereby resulting in the development of GaLV within two gibbons (S-76 and S-77).[3]
The last hypothesis is based on the sequence similarity of GaLV and retroviruses present within Southeast Asian bat species.[12] Mobile bat species are potential intermediate hosts of GALV as they can disperse rapidly over large geographical areas and have also been linked to several zoonotic diseases.[13]
Replication cycle
GaLV belongs to the retrovirus family which utilises an enzyme called reverse transcriptase in viral replication. Retroviruses have single stranded genomes (ssRNA) which undergoes reverse transcription to form double-stranded DNA (dsNDA) prior to proviral integration into the genome of the host cell. The GaLV replication cycle proceeds as follows:
- Binding: The first step of GaLV retroviral replication is the adsorption of adsorbate particles on the surface of human cells using receptor molecules SLC20A1 (GLVR-1, PIT-1) and SLC20A2 (GLVR-2, PIT-2).[14] Both molecules are cellular proteins (phosphate transporters).
- Entry into host cell: Then GaLV particles use these cell-surface proteins on the cell membrane, as specific receptors to enter their host cells.[15]
- Reverse transcription: The viral core then enters the cytoplasm of the target cell where the enzyme, reverse transcriptase, generates a complementary DNA strand from 3' to 5'.[15]
- Nuclear entry: The proviral integration of GaLV into the host genome requires entry into the nucleus of the target cell. However, GaLV is incapable of infecting non-dividing cells and therefore relies on the breakdown of the nuclear membrane during mitosis cell division for nuclear entry.[15]
- Replication: Once the proviral DNA enters the nucleus of the host cell, replication occurs via polypeptide synthesis and becomes integrated into the host genome.[15]
Viral resistance
Research published within the Retroviruses and Insights into Cancer Journal, highlights the potential of viral resistance within gibbon-apes, due to the partial proviral transcription of an intact envelope gene. The expression of the GaLV envelope gene was exhibited within an asymptomatic gibbon despite long term exposure to another highly viremic gibbon. Therefore, the expression of the GaLV envelope in the absence of replication-competent GaLV may have rendered the animal resistant to GaLV infection.[16] Furthermore, antibodies against the retrovirus was identified in gibbons without evidence of disease which suggests a natural immunological resistance to GaLV.[17]
Transmission
GaLV is an exogenous virus that is horizontally transmitted via contact with GaLV contaminated biomaterials such as urine and faces.[18] This is confirmed within hybrizidation assay which evidenced the lack of proviral genome within uninfected gibbons. Furthermore, experimental research conducted at the Comparative Oncology Laboratory demonstrates the "horizontal transmission of GaLV within a 14-month-old uninfected gibbon which contracted GaLV within six weeks of exposure to viremic individuals." Furthermore, GaLV is also transmitted prenatally via parent-progeny transmission in utero, of which offspring exhibit a large quantity of proviral DNA in opposed to postnatal transmission.[5]
Signs and symptoms
Conditions associated with GALV include neoplastic syndromes leading to susceptible secondary and often fatal diseases including; malignant lymphoma, lymphoblastic leukemia, osteoporosis and granulocytic leukemia. In cases of granulocytic leukemia, increased granulocytes in the peripheral blood infiltrated bone marrow and liver lymph nodes, causing a greenish hue (chlorosis) within these tissues.[17] Pathology study published by Kawakami et al in 1980, identifies the development of chronic granulocytic leukemia within young GaLV infected gibbons after latency periods of 5–11 months. Additionally, the introduction of GaLV into 14-month-old gibbons, demonstrated the production of neutralising antibodies which enabled individuals to remain asymptomatic and free of hematopoietic disease, thereby demonstrating the host's immune response to GaLV infection.[8]
Gammaretrovirus outbreaks
Koala retrovirus (KoRV)
KoRV belongs to the gammaretrovirus genus and is closely related to GaLV with an 80% nucleotide similarity.[19] The retrovirus is isolated from lymphomas and leukemias, present within infected captive and free-living koala populations in Australasia.[20] Accordingly, a study published within the journal of virology, Molecular Dynamics and Mode of Transmission of Koala Retrovirus as It Invades and Spreads through a Wild Queensland Koala Population, highlights that 80% of koalas that developed neoplasia was also KoRV-B positive, thereby linking the widespread infection of leukemia and lymphoma to KoRV. At present, KoRV is the only retroviral that induces germ-line infections and therefore presents the opportunity for scientists to understand the processes regulating retrovirus endogenization.[21]
9 subtypes of KoRV have been identified, with the primary strains being; KoRV-A, KoRV-B and KoRV-J, which induces immodulation resulting in neoplastic syndromes and chlamydiosis. Moreover, the study demonstrated the diseases associated with KoRV-B including; developed abdominal lymphoma, a nonspecified proliferative/bone marrow condition, osteochondroma and mesothelioma.[22] Nature by Tarlington and colleagues, provides epidemiological evidence that germline infections are present in populations found in Queensland, yet some individuals in Southern Australia lack the provirus, suggesting that retroviral endogenization began in Northern Australia between the last 100 to 200 years.[21] Pathology study of the endogenizing integration of KoRV-A into the host's genome is essential in developing a therapeutic vaccine which decreases the incidence rate of 3% per year.[23][22]
Feline leukaemia virus (FeLV)
FeLV is an oncogenic gammaretrovirus belonging to the orthoretrovirinae subfamily and retroviridae family.[24] First discovered in 1964 within a cluster of cats with lymphosarcoma. FeLV is identified as the infectious agent causing immunomodulation within bone marrow and the immune system, which renders infected cats susceptible to a variety of secondary and opportunistic infections.[25] Associated diseases of FeLV include; lymphoma, non-regenerative anemias and thymic degenerative disease.[26] Currently, the prevalence of FeLV has decreased since the 1970s and 1980s, due to veterinary interventions, vaccination, biosecurity protocols and quarantine or euthanasia of infected animals.[27] Accurate blood testing procedures revolving around the detection of FeLV P27 enables diagnosis via two methods; enzyme-linked immunosorbent assay (ELISA), which detects the presence of free FeLV particles that are found in the bloodstream and indirect immunofluorescent antibody assay (IFA), which detects the presence of retroviral particles within white blood cells.[28]
FeLV is horizontally and vertically transmitted through biomaterials; saliva, blood, breast milk, urine and feces. Furthermore, transmission can also occur postnatally or prenatally within parent-progeny relationships. The potency of parasitic fleas as a viral vector for FeLV was identified in 2003, which confirmed horizontal transmission of FeLV without close contact with infected individuals.[29] Furthermore, the three strains of FeLV are A,B,C. FeLV-A is the least pathogenic strain that is transmittable in nature especially within unvaccinated animals.[30] Contrarily, FeLV-B is derived via recombination of exogenous FeLV-A with endogenous sequences (enFeLV) whilst the limited research into the origins of FeLV-C leans towards recombination/ or mutation.[31]
Porcine endogenous retrovirus (PERV)
PERV was first described in 1970, belonging to the gammaretrovirus genus, Orthoretrovirinae subfamily and Retroviridae family,.[32] PERV is categorised into three replication competent subtypes: PERV-A, PERV-B and PERV-C. PERV-A and PERV-B are polytropic viruses which are capable of infecting humans and porcine cells, whereas PERV-C is an ecotropic virus which effects only porcine cells.[33] The cross-species transmission of PERV's in human cells have been demonstrated in vitro which raises concern regarding the xenotransplantation of porcine cells, tissues and organs.[33] However, diagnosis of PERV in vivo has not occurred within; recipients of pig nerve cells or skin grafts, patients with porcine-based liver or pancreatic xenografts, and butchers in contact with porcine tissue.[32]
In medicine
GaLV envelope protein
GaLV Envelope Protein has biomedical significance due to its utility as a viral vector in cancer gene therapy and gene transfer.[20] Retroviral vectors are used in ex vivo gene therapy, which involves the modification of cells in vitro, to replace genes that code for dysfunctional proteins. The inserted gene undergoes transcription and translation within the nucleus and ribosome of the host cell producing "normal" secretable proteins.[34] The earliest retroviral vectors were based on the Moloney murine leukemia virus (MMLV) which when pseudotyped with GaLV envelope protein, enabled gene transfer to various host cells.[35] Furthermore, the development of "hybrid murine amphotropic viral envelope with the extracellular domains of GALV also helps to increase the cell infection rate within the host during gene therapy."[36][37]
Gene transfer is dependent on the relationship between receptor expression and transduction efficiency. Human T-lymphocytes have two surface receptors (GLVR-1 and GLVR-2) that detect the presence of GaLV. Furthermore, Lam et al evidenced the 8 fold greater expression of GLVR-1 than GLVR-2, which demonstrates that human T lymphocyte gene transfer methods should utilise the GaLV envelope protein that binds to the GLVR-1 surface receptor.[38] However, because gammaretroviruses are incapable of infecting non-dividing cells, the utility of GaLV envelope protein in gene transfer is being superseded by lentiviral vectors.[35]
References
- ↑ S, Delassus; P, Sonigo; S, Wain-Hobson (November 1989). "Genetic Organization of Gibbon Ape Leukemia Virus". Virology. 173 (1): 205–13. doi:10.1016/0042-6822(89)90236-5. PMID 2683360.
- 1 2 3 4 5 J, McKee; N, Clark; F, Shapter; G, Simmons (April 2017). "A New Look at the Origins of Gibbon Ape Leukemia Virus". Virus Genes. 53 (2): 165–172. doi:10.1007/s11262-017-1436-0. PMID 28220345. S2CID 28786457.
- 1 2 3 4 5 6 7 Brown, Katherine; Tarlinton, Rachael E. (January 2017). "Is gibbon ape leukaemia virus still a threat?" (PDF). Mammal Review. 47 (1): 53–61. doi:10.1111/mam.12079.
- 1 2 3 4 Murphy, Hayley Weston; Switzer, William M. (2008-01-01), Fowler, Murray E.; Miller, R. Eric (eds.), "Chapter 31 - Occupational Exposure to Zoonotic Simian Retroviruses: Health and Safety Implications for Persons Working with Nonhuman Primates", Zoo and Wild Animal Medicine (Sixth Edition), W.B. Saunders, pp. 251–264, ISBN 978-1-4160-4047-7, retrieved 2020-02-02
- 1 2 Kawakami, Thomas (1978-10-04). "Natural Transmission of Gibbon Leukemia Virus". Journal of the National Cancer Institute. 61 (4): 1113–5. PMID 212567 – via Google Booka.
- ↑ "Complete genome of all strains of the gibbon ape leukemia virus sequenced". ScienceDaily. Retrieved 2020-02-09.
- ↑ "Virologists unravel mystery of late 20th century gibbon leukaemia outbreak". ScienceDaily. Retrieved 2020-02-06.
- 1 2 3 Hausen, Harald zur (2007-09-24). Infections Causing Human Cancer. John Wiley & Sons. ISBN 978-3-527-60929-1.
- ↑ Reitz, M S; wong-Staal, F; Haseltine, W A; Kleid, D G; Trainor, C D; Gallagher, R E; Gallo, R C (January 1979). "Gibbon ape leukemia virus-Hall's Island: new strain of gibbon ape leukemia virus". Journal of Virology. 29 (1): 395–400. doi:10.1128/JVI.29.1.395-400.1979. ISSN 0022-538X. PMC 353141. PMID 219232.
- ↑ Burke, Mark; Ptito, Maurice (2018-05-30). Primates. BoD – Books on Demand. ISBN 978-1-78923-216-5.
- ↑ Simmons, Greg; Clarke, Daniel; McKee, Jeff; Young, Paul; Meers, Joanne (2014-09-24). Roca, Alfred L. (ed.). "Discovery of a Novel Retrovirus Sequence in an Australian Native Rodent (Melomys burtoni): A Putative Link between Gibbon Ape Leukemia Virus and Koala Retrovirus". PLOS ONE. 9 (9): e106954. Bibcode:2014PLoSO...9j6954S. doi:10.1371/journal.pone.0106954. ISSN 1932-6203. PMC 4175076. PMID 25251014.
- ↑ J, Denner (2016-12-20). "Transspecies Transmission of Gammaretroviruses and the Origin of the Gibbon Ape Leukaemia Virus (GaLV) and the Koala Retrovirus (KoRV)". Viruses. 8 (12): 336. doi:10.3390/v8120336. PMC 5192397. PMID 27999419.
- ↑ Alfano, Niccolò; Michaux, Johan; Morand, Serge; Aplin, Ken; Tsangaras, Kyriakos; Löber, Ulrike; Fabre, Pierre-Henri; Fitriana, Yuli; Semiadi, Gono; Ishida, Yasuko; Helgen, Kristofer M. (2016-08-26). "Endogenous Gibbon Ape Leukemia Virus Identified in a Rodent (Melomys burtoni subsp.) from Wallacea (Indonesia)". Journal of Virology. 90 (18): 8169–8180. doi:10.1128/JVI.00723-16. ISSN 0022-538X. PMC 5008096. PMID 27384662.
- ↑ Liu, Meihong; Eiden, Maribeth V. (2011-07-05). "The receptors for gibbon ape leukemia virus and amphotropic murine leukemia virus are not downregulated in productively infected cells". Retrovirology. 8 (1): 53. doi:10.1186/1742-4690-8-53. ISSN 1742-4690. PMC 3136417. PMID 21729311.
- 1 2 3 4 Nisole, Sébastien; Saïb, Ali (2004-05-14). "Early steps of retrovirus replicative cycle". Retrovirology. 1: 9. doi:10.1186/1742-4690-1-9. ISSN 1742-4690. PMC 421752. PMID 15169567.
- ↑ Dudley, Jaquelin (2010-10-22). Retroviruses and Insights into Cancer. Springer Science & Business Media. ISBN 978-0-387-09581-3.
- 1 2 Lowenstine, Linda J.; McManamon, Rita; Terio, Karen A. (2018-01-01), Terio, Karen A.; McAloose, Denise; Leger, Judy St. (eds.), "Chapter 15 - Apes", Pathology of Wildlife and Zoo Animals, Academic Press, pp. 375–412, ISBN 978-0-12-805306-5, retrieved 2020-02-09
- ↑ Murphy, Hayley Weston; Switzer, William M. (2008-01-01), Fowler, Murray E.; Miller, R. Eric (eds.), "Chapter 31 - Occupational Exposure to Zoonotic Simian Retroviruses: Health and Safety Implications for Persons Working with Nonhuman Primates", Zoo and Wild Animal Medicine (Sixth Edition), W.B. Saunders, pp. 251–264, ISBN 978-1-4160-4047-7, retrieved 2020-02-09
- ↑ Alfano, Niccolò; Michaux, Johan; Morand, Serge; Aplin, Ken; Tsangaras, Kyriakos; Löber, Ulrike; Fabre, Pierre-Henri; Fitriana, Yuli; Semiadi, Gono; Ishida, Yasuko; Helgen, Kristofer M. (2016-09-15). "Endogenous Gibbon Ape Leukemia Virus Identified in a Rodent (Melomys burtoni subsp.) from Wallacea (Indonesia)". Journal of Virology. 90 (18): 8169–8180. doi:10.1128/JVI.00723-16. ISSN 0022-538X. PMID 27384662.
- 1 2 Denner, Joachim; Young, Paul R (2013-10-23). "Koala retroviruses: characterization and impact on the life of koalas". Retrovirology. 10: 108. doi:10.1186/1742-4690-10-108. ISSN 1742-4690. PMC 4016316. PMID 24148555.
- 1 2 Stoye, Jonathan P (2006). "Koala retrovirus: a genome invasion in real time". Genome Biology. 7 (11): 241. doi:10.1186/gb-2006-7-11-241. ISSN 1465-6906. PMC 1794577. PMID 17118218.
- 1 2 Quigley, Bonnie L.; Ong, Vanissa A.; Hanger, Jonathan; Timms, Peter (2018-03-01). "Molecular Dynamics and Mode of Transmission of Koala Retrovirus as It Invades and Spreads through a Wild Queensland Koala Population". Journal of Virology. 92 (5). doi:10.1128/JVI.01871-17. ISSN 0022-538X. PMC 5809739. PMID 29237837.
- ↑ Olagoke, O.; Quigley, B. L.; Eiden, M. V.; Timms, P. (2019-08-27). "Antibody response against koala retrovirus (KoRV) in koalas harboring KoRV-A in the presence or absence of KoRV-B". Scientific Reports. 9 (1): 12416. doi:10.1038/s41598-019-48880-0. ISSN 2045-2322. PMC 6711960. PMID 31455828.
- ↑ "Retroviridae". www.uniprot.org. Retrieved 2020-02-16.
- ↑ Hardy, W. D.; Hess, P. W.; MacEwen, E. G.; McClelland, A. J.; Zuckerman, E. E.; Essex, M.; Cotter, S. M.; Jarrett, O. (February 1976). "Biology of feline leukemia virus in the natural environment". Cancer Research. 36 (2 pt 2): 582–588. ISSN 0008-5472. PMID 175919.
- ↑ O’Connor, Thomas P.; Lawrence, John; Andersen, Philip; Leathers, Valerie; Workman, Erwin (2013-01-01), Wild, David (ed.), "Chapter 8.1 - Immunoassay Applications in Veterinary Diagnostics", The Immunoassay Handbook (Fourth Edition), Elsevier, pp. 623–645, ISBN 978-0-08-097037-0, retrieved 2020-02-16
- ↑ Westman, Mark; Norris, Jacqueline; Malik, Richard; Hofmann-Lehmann, Regina; Harvey, Andrea; McLuckie, Alicia; Perkins, Martine; Schofield, Donna; Marcus, Alan; McDonald, Mike; Ward, Michael (2019-05-31). "The Diagnosis of Feline Leukaemia Virus (FeLV) Infection in Owned and Group-Housed Rescue Cats in Australia". Viruses. 11 (6): 503. doi:10.3390/v11060503. ISSN 1999-4915. PMC 6630418. PMID 31159230.
- ↑ "Feline Leukemia Virus". Cornell University College of Veterinary Medicine. 2017-10-11. Retrieved 2020-02-18.
- ↑ Vobis, M.; d'Haese, J.; Mehlhorn, H.; Mencke, N. (2003). "Signing into eresources, The University of Sydney Library". Parasitology Research. 91 (6): 467–70. doi:10.1007/s00436-003-0949-8. PMID 14557874. S2CID 23898163.
- ↑ Bolin, Lisa L.; Ahmad, Shamim; Lobelle-Rich, Patricia A.; Ooms, Tara G.; Alvarez-Hernandez, Xavier; Didier, Peter J.; Levy, Laura S. (October 2013). "The Surface Glycoprotein of Feline Leukemia Virus Isolate FeLV-945 Is a Determinant of Altered Pathogenesis in the Presence or Absence of the Unique Viral Long Terminal Repeat". Journal of Virology. 87 (19): 10874–10883. doi:10.1128/JVI.01130-13. ISSN 0022-538X. PMC 3807393. PMID 23903838.
- ↑ Chang, Zongli; Pan, Judong; Logg, Christopher; Kasahara, Noriyuki; Roy-Burman, Pradip (September 2001). "A Replication-Competent Feline Leukemia Virus, Subgroup A (FeLV-A), Tagged with Green Fluorescent Protein Reporter Exhibits In Vitro Biological Properties Similar to Those of the Parental FeLV-A". Journal of Virology. 75 (18): 8837–8841. doi:10.1128/JVI.75.18.8837-8841.2001. ISSN 0022-538X. PMC 115128. PMID 11507228.
- 1 2 Łopata, Krzysztof; Wojdas, Emilia; Nowak, Roman; Łopata, Paweł; Mazurek, Urszula (2018-04-11). "Porcine Endogenous Retrovirus (PERV) – Molecular Structure and Replication Strategy in the Context of Retroviral Infection Risk of Human Cells". Frontiers in Microbiology. 9: 730. doi:10.3389/fmicb.2018.00730. ISSN 1664-302X. PMC 5932395. PMID 29755422.
- 1 2 Denner, Joachim (2016-08-03). "How Active Are Porcine Endogenous Retroviruses (PERVs)?". Viruses. 8 (8): 215. doi:10.3390/v8080215. ISSN 1999-4915. PMC 4997577. PMID 27527207.
- ↑ Hunter, Jacqueline E.; Ramos, Linnet; Wolfe, John H. (2017-01-01), "Viral Vectors in the CNS☆", Reference Module in Neuroscience and Biobehavioral Psychology, Elsevier, ISBN 978-0-12-809324-5, retrieved 2020-02-17
- 1 2 Cooray, Samantha; Howe, Steven J.; Thrasher, Adrian J. (2012-01-01), Friedmann, Theodore (ed.), "Chapter three - Retrovirus and Lentivirus Vector Design and Methods of Cell Conditioning", Methods in Enzymology, Gene Transfer Vectors for Clinical Application, Academic Press, vol. 507, pp. 29–57, retrieved 2020-02-17
- ↑ Worgall, Stefan; Crystal, Ronald G. (2014-01-01), Lanza, Robert; Langer, Robert; Vacanti, Joseph (eds.), "Chapter 34 - Gene Therapy", Principles of Tissue Engineering (Fourth Edition), Academic Press, pp. 657–686, ISBN 978-0-12-398358-9, retrieved 2020-02-17
- ↑ Fischer, Alain; Hacein-Bey-Abina, Salima; Cavazzana-Calvo, Marina (2014-01-01), Etzioni, Amos; Ochs, Hans D. (eds.), "Chapter 26 - How Primary Immunodeficiencies Have Made Gene Therapy a Reality", Primary Immunodeficiency Disorders, Academic Press, pp. 327–339, ISBN 978-0-12-407179-7, retrieved 2020-02-17
- ↑ Lam, John S.; Reeves, Mark E.; Cowherd, Robert; Rosenberg, Steven A.; Hwu, Patrick (August 1996). "Improved Gene Transfer into Human Lymphocytes Using Retroviruses with the Gibbon Ape Leukemia Virus Envelope". Human Gene Therapy. 7 (12): 1415–1422. doi:10.1089/hum.1996.7.12-1415. ISSN 1043-0342. PMID 8844200.
