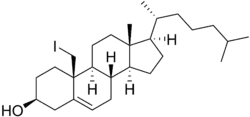
Iodocholesterol
 | |
| Clinical data | |
|---|---|
| Other names | Iodocholesterol; 19-Iodocholesterol; Iodocholesterol (131I); 19-Iodocholest-5-en-3β-ol |
| ATC code | |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.048.618 |
| Chemical and physical data | |
| Formula | C27H45IO |
| Molar mass | 512.560 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
Iodocholesterol, or 19-iodocholesterol, also as iodocholesterol (131I) (INN), is a derivative of cholesterol with an iodine atom in the C19 position and a radiopharmaceutical.[1][2] When the iodine atom is a radioactive isotope (iodine-125 or iodine-131), it is used as an adrenal cortex radiocontrast agent in the diagnosis of patients suspected of having Cushing's syndrome, hyperaldosteronism, pheochromocytoma, and adrenal remnants following total adrenalectomy.[1][2]
References
- 1 2 Elks J (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 692–. ISBN 978-1-4757-2085-3.
- 1 2 Clark OH, American Cancer Society (2003). Endocrine Tumors. PMPH-USA. pp. 124–. ISBN 978-1-55009-134-2.
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.