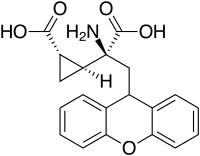
LY-341495
 | |
| Clinical data | |
|---|---|
| Other names | (2S)-2-Amino-2-[(1S,2S)-2-carboxycycloprop-1-yl]-3-(xanth-9-yl)propanoic acid |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| ChemSpider | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C20H19NO5 |
| Molar mass | 353.374 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
LY-341495 is a research drug developed by the pharmaceutical company Eli Lilly, which acts as a potent and selective orthosteric antagonist for the group II metabotropic glutamate receptors (mGluR2/3).[1][2][3][4]
It is used in scientific research in several different areas, showing antidepressant effects in animal models,[5][6][7][8] increasing the behavioural effects of hallucinogenic drugs in animal tests,[9][10][11][12] and increasing the analgesic effects of μ-opioid agonists,[13][14] as well as modulating dopamine receptor function.[15][16][17]
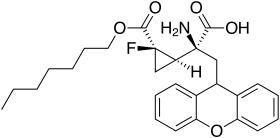
The 1-fluorocyclopropane analog has a superior pharmacokinetic profile and similar mGluR2/3 affinity, and making a prodrug from this with the heptyl ester increases bioavailability still further.[18]
See also
References
- ↑ Ornstein PL, Bleisch TJ, Arnold MB, Kennedy JH, Wright RA, Johnson BG, Tizzano JP, Helton DR, Kallman MJ, Schoepp DD, Hérin M (January 1998). "2-substituted (2SR)-2-amino-2-((1SR,2SR)-2-carboxycycloprop-1-yl)glycines as potent and selective antagonists of group II metabotropic glutamate receptors. 2. Effects of aromatic substitution, pharmacological characterization, and bioavailability". Journal of Medicinal Chemistry. 41 (3): 358–78. doi:10.1021/jm970498o. PMID 9464367.
- ↑ Kingston AE, Ornstein PL, Wright RA, Johnson BG, Mayne NG, Burnett JP, Belagaje R, Wu S, Schoepp DD. "LY341495 is a nanomolar potent and selective antagonist of group II metabotropic glutamate receptors". Neuropharmacology. 37 (1): 1–12. doi:10.1016/s0028-3908(97)00191-3. PMID 9680254.
- ↑ Fitzjohn SM, Bortolotto ZA, Palmer MJ, Doherty AJ, Ornstein PL, Schoepp DD, Kingston AE, Lodge D, Collingridge GL (December 1998). "The potent mGlu receptor antagonist LY341495 identifies roles for both cloned and novel mGlu receptors in hippocampal synaptic plasticity". Neuropharmacology. 37 (12): 1445–58. doi:10.1016/s0028-3908(98)00145-2. PMID 9886667.
- ↑ Johnson BG, Wright RA, Arnold MB, Wheeler WJ, Ornstein PL, Schoepp DD (October 1999). "[3H]-LY341495 as a novel antagonist radioligand for group II metabotropic glutamate (mGlu) receptors: characterization of binding to membranes of mGlu receptor subtype expressing cells". Neuropharmacology. 38 (10): 1519–29. doi:10.1016/s0028-3908(99)00053-2. PMID 10530814.
- ↑ Pilc A, Chaki S, Nowak G, Witkin JM (March 2008). "Mood disorders: regulation by metabotropic glutamate receptors". Biochemical Pharmacology. 75 (5): 997–1006. doi:10.1016/j.bcp.2007.09.021. PMID 18164691.
- ↑ Matrisciano F, Panaccione I, Zusso M, Giusti P, Tatarelli R, Iacovelli L, Mathé AA, Gruber SH, Nicoletti F, Girardi P (August 2007). "Group-II metabotropic glutamate receptor ligands as adjunctive drugs in the treatment of depression: a new strategy to shorten the latency of antidepressant medication?". Molecular Psychiatry. 12 (8): 704–6. doi:10.1038/sj.mp.4002005. PMID 17653204.
- ↑ Koike H, Chaki S (September 2014). "Requirement of AMPA receptor stimulation for the sustained antidepressant activity of ketamine and LY341495 during the forced swim test in rats". Behavioural Brain Research. 271: 111–5. doi:10.1016/j.bbr.2014.05.065. PMID 24909673.
- ↑ Lepack AE, Bang E, Lee B, Dwyer JM, Duman RS (December 2016). "Fast-acting antidepressants rapidly stimulate ERK signaling and BDNF release in primary neuronal cultures". Neuropharmacology. 111: 242–252. doi:10.1016/j.neuropharm.2016.09.011. PMC 5075989. PMID 27634096.
- ↑ Gewirtz JC, Marek GJ (November 2000). "Behavioral evidence for interactions between a hallucinogenic drug and group II metabotropic glutamate receptors". Neuropsychopharmacology. 23 (5): 569–76. doi:10.1016/S0893-133X(00)00136-6. PMID 11027922.
- ↑ Benneyworth MA, Xiang Z, Smith RL, Garcia EE, Conn PJ, Sanders-Bush E (August 2007). "A selective positive allosteric modulator of metabotropic glutamate receptor subtype 2 blocks a hallucinogenic drug model of psychosis". Molecular Pharmacology. 72 (2): 477–84. doi:10.1124/mol.107.035170. PMID 17526600.
- ↑ Carbonaro TM, Eshleman AJ, Forster MJ, Cheng K, Rice KC, Gatch MB (January 2015). "The role of 5-HT2A, 5-HT 2C and mGlu2 receptors in the behavioral effects of tryptamine hallucinogens N,N-dimethyltryptamine and N,N-diisopropyltryptamine in rats and mice". Psychopharmacology. 232 (1): 275–84. doi:10.1007/s00213-014-3658-3. PMC 4282596. PMID 24985890.
- ↑ Moreno JL, González-Maeso J. "Crosstalk Between 5-HT2A and mGlu2 Receptors: Implications in Schizophrenia and Its Treatment". 5-HT2A Receptors in the Central Nervous System. The Receptors. Vol. 32. pp. 147–189. doi:10.1007/978-3-319-70474-6_7. ISBN 978-3-319-70472-2.
- ↑ Fischer BD, Miller LL, Henry FE, Picker MJ, Dykstra LA (June 2008). "Increased efficacy of micro-opioid agonist-induced antinociception by metabotropic glutamate receptor antagonists in C57BL/6 mice: comparison with (-)-6-phosphonomethyl-deca-hydroisoquinoline-3-carboxylic acid (LY235959)". Psychopharmacology. 198 (2): 271–8. doi:10.1007/s00213-008-1130-y. PMID 18392754.
- ↑ Fischer BD, Zimmerman EI, Picker MJ, Dykstra LA (February 2008). "Morphine in combination with metabotropic glutamate receptor antagonists on schedule-controlled responding and thermal nociception". The Journal of Pharmacology and Experimental Therapeutics. 324 (2): 732–9. doi:10.1124/jpet.107.131417. PMID 17982001.
- ↑ O'Neill MF, Heron-Maxwell C, Conway MW, Monn JA, Ornstein P (October 2003). "Group II metabotropic glutamate receptor antagonists LY341495 and LY366457 increase locomotor activity in mice". Neuropharmacology. 45 (5): 565–74. doi:10.1016/S0028-3908(03)00232-6. PMID 12941370.
- ↑ Chi H, Jang JK, Kim JH, Vezina P (October 2006). "Blockade of group II metabotropic glutamate receptors in the nucleus accumbens produces hyperlocomotion in rats previously exposed to amphetamine". Neuropharmacology. 51 (5): 986–92. doi:10.1016/j.neuropharm.2006.06.008. PMID 16901517.
- ↑ Yoon HS, Jang JK, Kim JH (September 2008). "Blockade of group II metabotropic glutamate receptors produces hyper-locomotion in cocaine pre-exposed rats by interactions with dopamine receptors". Neuropharmacology. 55 (4): 555–9. doi:10.1016/j.neuropharm.2008.07.012. PMID 18675831.
- ↑ Sakagami K, Yasuhara A, Chaki S, Yoshikawa R, Kawakita Y, Saito A, Taguchi T, Nakazato A (April 2008). "Synthesis, in vitro pharmacology, and pharmacokinetic profiles of 2-[1-amino-1-carboxy-2-(9H-xanthen-9-yl)-ethyl]-1-fluorocyclopropanecarboxylic acid and its 6-heptyl ester, a potent mGluR2 antagonist". Bioorganic & Medicinal Chemistry. 16 (8): 4359–66. doi:10.1016/j.bmc.2008.02.066. PMID 18348906.