Myringotomy
| Myringotomy | |
|---|---|
| ICD-9-CM | 20.020.0120.09 |
A myringotomy is a surgical procedure in which an incision is created in the eardrum (tympanic membrane) to relieve pressure caused by excessive buildup of fluid, or to drain pus from the middle ear. A tympanostomy tube may be inserted through the eardrum to keep the middle ear aerated for a prolonged time and to prevent reaccumulation of fluid. Without the insertion of a tube, the incision usually heals spontaneously within two to three weeks. Depending on the type, the tube is either naturally extruded in 6 to 12 months or removed during a minor procedure.[1]
Those requiring myringotomy usually have an obstructed or dysfunctional eustachian tube that is unable to perform drainage or ventilation in its usual fashion. Before the invention of antibiotics, myringotomy without tube placement was also used as a major treatment of severe acute otitis media (middle ear infection).[1]
Nomenclature
The words myringotomy, tympanotomy, tympanostomy, and tympanocentesis overlap in meaning. The first two are always synonymous, and the third is often used synonymously.[2] The core idea with each is cutting a hole in the eardrum to allow fluid to pass through it. Sometimes a distinction is drawn between myringotomy/tympanotomy and tympanostomy, in parallel with the general distinction between an -otomy (cutting) and an -ostomy (creating a stoma with some degree of permanence or semipermanence). In this distinction, only a tympanostomy involves tympanostomy tubes and creates a semipermanent stoma. This distinction in usage is not always made. The word tympanocentesis specifies that centesis (aspiration for sampling) is being done.
Etymologically, myringotomy (myringo-, from Latin myringa "eardrum",[3] + -tomy) and tympanotomy (tympano- + -tomy) both mean "eardrum cutting", and tympanostomy (tympano- + -stomy means "making an eardrum stoma".
History
In 1649, Jean Riolan the Younger accidentally pierced a patient's ear drum while cleaning it with an ear spoon. Surprisingly, the patient's hearing improved. There are also reports from the 17th and 18th centuries describing separate experiments exploring the function of the ear drum.[4] In particular, the animal experiments of Thomas Willis were expanded upon by Sir Astley Cooper, who presented two papers to the Royal Society in 1801 on his observations that myringotomy could improve hearing.[5] First, he showed that two patients with perforations of both eardrums could hear perfectly well, despite conventional wisdom that this would result in deafness. Second, he demonstrated that deafness caused by obstruction of the Eustachian tube could be relieved by myringotomy, which equalized the pressure on each side of the tympanic membrane.
Widespread inappropriate use of the procedure later led to it falling out of use. However, it was reintroduced by Hermann Schwartze in the 19th century. An inherent problem became recognized, namely the tendency of the tympanic membrane to heal spontaneously and rapidly, reversing the beneficial effects of the perforation. In order to prevent this, a tympanostomy tube, initially made of gold foil, was placed through the incision to prevent it from closing. Ádám Politzer, a Hungarian-born otologist practicing in Vienna, experimented with rubber in 1886. The vinyl tube used today was introduced by Beverly Armstrong in 1954.[6]
Indications
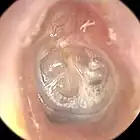
There are numerous indications for tympanostomy in the pediatric age group,[1][7] the most frequent including chronic otitis media with effusion (OME) which is unresponsive to antibiotics, and recurrent otitis media. Adult indications[1][8][9] differ somewhat and include Eustachian tube dysfunction with recurrent signs and symptoms, including fluctuating hearing loss, vertigo, tinnitus, and a severe retraction pocket in the tympanic membrane. Recurrent episodes of barotrauma, especially with flying, diving, or hyperbaric chamber treatment, may merit consideration.
Procedure
Myringotomy is usually performed as an outpatient procedure. General anesthesia is preferred in children, while local anesthesia suffices for adults. The ear is washed and a small incision made in the eardrum. Any fluid that is present is then aspirated, the tube of choice inserted, and the ear packed with cotton to control any slight bleeding that might occur. This is known as conventional (or cold knife) myringotomy and usually heals in one to two days.[10]
A new variation (called tympanolaserostomy or laser-assisted tympanostomy) uses a CO2 laser, and is performed with a computer-driven laser and a video monitor to pinpoint a precise location for the hole. The laser takes one-tenth of a second to create the opening, without damaging surrounding skin or other structures. This perforation remains patent for several weeks and provides ventilation of the middle ear without the need for tube placement.[11]
Though laser myringotomies maintain patency slightly longer than cold-knife myringotomies (two to three weeks for laser and two to three days for cold knife without tube insertion),[12] they have not proven to be more effective in the management of effusion. One randomized controlled study found that laser myringotomies are safe but less effective than ventilation tube in the treatment of chronic OME.[13] Multiple occurrences in children, a strong history of allergies in children, the presence of thick mucoid effusions, and history of tympanostomy tube insertion in adults, make it likely that laser tympanostomy will be ineffective.[10]
Various tympanostomy tubes are available. Traditional metal tubes have been replaced by more popular silicon, titanium, polyethylene, gold, stainless steel, or fluoroplastic tubes. More recent ones are coated with antibiotics and phosphorylcholine.
Aftercare
There is little scientific evidence to guide the care of the ear after tubes have been inserted. A single, randomized trial found statistical benefit to using ear protective devices when swimming although the size of the benefit was quite small.[14] In the absence of strong evidence, general opinion has been against the use of ear protection devices. However, protection such as cotton covered with petroleum jelly, ear plugs, or ear putty is recommended for swimming in dirty water (lakes, rivers, oceans, or non-chlorinated pools) to prevent ear infections. For bathing, shampooing, or surface-water swimming in chlorinated pools, no ear protection is recommended.
Complications
The placement of tubes is not a cure. If middle ear disease has been severe or prolonged enough to justify tube placement, there is a strong possibility that the child will continue to have episodes of middle ear inflammation or fluid collection. There may be early drainage through the tube (tube otorrhea) in about 15% of patients in the first two weeks after placement, and developing in 25% more than three months after insertion, although usually not a longterm problem.[15] Otorrhea is considered to be secondary to bacterial colonization. The most commonly isolated organism is Pseudomonas aeruginosa, while the most troublesome is Methicillin-resistant Staphylococcus aureus (MRSA). Some practitioners use topical antibiotic drops in the postoperative period, but research shows that this practice does not eradicate the bacterial biofilm.[1] A laboratory study showed that tubes covered in the antibiotic vancomycin prevented in-vitro formation of MRSA biofilm as compared to noncoated ones,[16] although no study has been conducted on humans yet. Comparing phosphorylcholine-coated fluoroplastic tympanostomy tubes to uncoated fluoroplastic tympanostomy tubes showed no statistically significant difference in the incidence of post-operative otorrhea, tube blockage, or extrusion.[17]
Efficacy
Evidence suggests that tympanostomy tubes only offer a short-term hearing improvement in children with simple OME who have no other serious medical problems. No effect on speech and language development has yet been shown.[18]
A retrospective study of success rates in 96 adults and 130 children with otitis media treated with CO2 laser myringotomy showed about a 50% cure rate at six months in both groups.[10] To date, there have been no published systematic reviews.
Balloon dilation eustachian tuboplasty (BDET), a new treatment, has proven to be effective in treating OME secondary to eustachian tube dysfunction.[19][20][21] However, the number of patients in the studies cited, 22 and 8 respectively and 18 in the tympanometric study, is extremely small and simply points to the need for large, well-controlled studies.
See also
- Myringoplasty
- Otitis media
- List of surgeries by type
References
- 1 2 3 4 5 Smith N, Greinwald JR (2011). "To tube or not to tube: indications for myringotomy with tube placement". Current Opinion in Otolaryngology & Head and Neck Surgery. 19 (5): 363–366. doi:10.1097/MOO.0b013e3283499fa8. PMID 21804383. S2CID 3027628.
- ↑ Elsevier, Dorland's Illustrated Medical Dictionary, Elsevier.
- ↑ "myringotomy". Mosby's Medical Dictionary (8th ed.). Elsevier. 2009.
- ↑ Brusis T, Luckhaupt H (March 1995). "Der Trommelfellstich: Zur Geschichte von Parazentese und Paukenröhrchen" [Perforation of the ear drum. On the history of paracentesis and grommet insertion]. Laryngo-Rhino-Otologie (in German). 75 (3): 178–83. doi:10.1055/s-2007-997559. PMID 8652036.
- ↑ McGovern FH. Sir Astley Cooper and his otological papers. Ann Otol Rhinol Laryngol. 1984 Nov-Dec;93(6 Pt 1):531-3. doi: 10.1177/000348948409300601. PMID: 6391339
- ↑ Rimmer J, Giddings CE, Weir N (October 2007). "History of myringotomy and grommets". The Journal of Laryngology and Otology. 121 (10): 911–6. doi:10.1017/S0022215107009176. PMID 17559714. S2CID 27874209.
- ↑ American Academy of Family Physicians; American Academy of Otolaryngology–Head and Neck Surgery; American Academy of Pediatrics Subcommittee on Otitis Media With Effusion (May 2004). "Otitis media with effusion". Pediatrics. 113 (5): 1412–29. doi:10.1542/peds.113.5.1412. PMID 15121966.
- ↑ Kisser U, Gürkov R, Louza J, Schrötzlmair F, Adderson-Kisser C, Krause E (2012). "Comparison of characteristic of titanium and fluoropastic ventilation tubes in adults with healthy middle ears". Otology & Neurotology. 33 (6): 983–987. doi:10.1097/MAO.0b013e318259b70b. PMID 22772000. S2CID 33137759.
- ↑ "Ear tube insertion". MedlinePlus. U.S. National Library of Medicine.
- 1 2 3 Chang CW, Yang YW, Fu CY, Shiao AS (January 2012). "Differences between children and adults with otitis media with effusion treated with CO(2) laser myringotomy". Journal of the Chinese Medical Association. 75 (1): 29–35. doi:10.1016/j.jcma.2011.10.001. PMID 22240534.
- ↑ Lustig LR, Ingram A, Vidrine DM, Gould AR, Zeiders JW, Ow RA, Thompson CR, Moss JR, Mehta R, McClay JE, Brenski A, Gavin J, Waldman EH, Ansley J, Yen DM, Chadha NK, Murray MT, Kozak FK, York C, Brown DM, Grunstein E, Sprecher RC, Sherman DA, Schoem SR, Puchalski R, Hills S, Calzada A, Harfe D, England LJ, Syms CA 3rd. In-Office Tympanostomy Tube Placement in Children Using Iontophoresis and Automated Tube Delivery. Laryngoscope. 2020 May;130 Suppl 4(Suppl 4):S1-S9. doi: 10.1002/lary.28612. Epub 2020 Mar 11. PMID: 32160320; PMCID: PMC7187287.
- ↑ Prokopakis EP, Hajiioannou JK, Velegrakis GA, Christodoulou PN, Scordalakis C, Helidonis ES (Feb 25, 2002). "Duration of patency of laser-assisted tympanic membrane fenestration". Int J Pediatr Otorhinolaryngol. 62 (3): 207–14. doi:10.1016/s0165-5876(01)00613-9. PMID 11852122.
- ↑ Koopman JP, Reuchlin AG, Kummer EE, Boumans LJ, Rijntjes E, Hoeve LJ, Mulder PG, Blom HM (2004). "Laser myringotomy versus ventilation tubes in children with otitis media with effusion: a randomized trial". Laryngoscope. 114 (5): 844–9. doi:10.1097/00005537-200405000-00010. PMID 15126741. S2CID 73100585.
- ↑ Goldstein NA, Mandel EM, Kurs-Lasky M, Rockette HE, Casselbrandt ML (2005). "Water precautions and tympanostomy tubes: a randomized, controlled trial". Laryngoscope. 115 (2): 324–30. doi:10.1097/01.mlg.0000154742.33067.fb. PMID 15689760. S2CID 36426890.
- ↑ Kay DJ, Nelson M, Rosenfeld RM (April 2001). "Meta-analysis of tympanostomy tube sequelae". Otolaryngol Head Neck Surg. 124 (4): 374–80. doi:10.1067/mhn.2001.113941. PMID 11283489. S2CID 25287112.
- ↑ Jang CH, Park H, Cho YB, Choi CH (2010). "Effect of vancomycin-coated tympanostomy tubes on methicillin-resistant Staphylococcus aureus biofilm formation: In vitro study". The Journal of Laryngology & Otology. 124 (6): 594–598. doi:10.1017/S0022215109992672. PMID 20056010. S2CID 24655349.
- ↑ Hong P, Smith N, Johnson LB, Corsten G (2011). "A randomized double-blind controlled trial of phosphorylcholine-coated tympanostomy tube versus standard tympanostomy tube in children with recurrent acute and chronic otitis media". Laryngoscope. 121 (1): 214–219. doi:10.1002/lary.21156. PMID 21072756. S2CID 22624953.
- ↑ Browning GG, Rovers MM, Williamson I, Lous J, Burton MJ (2010). "Grommets (ventilation tubes) for hearing loss associated with otitis media with effusion in children". Cochrane Database of Systematic Reviews (10): CD001801. doi:10.1002/14651858.CD001801.pub3. PMID 20927726..
- ↑ McCoul ED, Anand VK (May–June 2012). "Eustachian tube balloon dilation surgery". International Forum of Allergy & Rhinology. 2 (3): 191–8. doi:10.1002/alr.21007. PMID 22253073. S2CID 20637776.
- ↑ Ockermann T, Reineke U, Upile T, Ebmeyer J, Sudhoff HH (July 2010). "Balloon dilatation eustachian tuboplasty: a clinical study". Laryngoscope. 120 (7): 1411–6. doi:10.1002/lary.20950. PMID 20564474. S2CID 7976041.
- ↑ Williams, Blair; Taylor, Benjamin A.; Clifton, Neil; Bance, Manohar (12 February 2016). "Balloon dilation of the eustachian tube: a tympanometric outcomes analysis". Journal of Otolaryngology - Head & Neck Surgery. BioMed Central. 45 (13): 13. doi:10.1186/s40463-016-0126-6. ISSN 1916-0216. PMC 4751715. PMID 26869258.