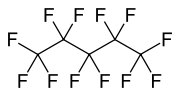
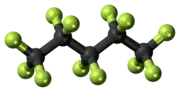
Perfluoropentane
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
Dodecafluoropentane[1] | |
| Other names
Perfluoropentane | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
Beilstein Reference |
1712388 |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.010.589 |
| EC Number |
|
| KEGG | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
InChI
| |
SMILES
| |
| Properties | |
Chemical formula |
C5F12 |
| Molar mass | 288.036 g·mol−1 |
| Density | 1.63 g/mL (liquid, 25 °C) [2] 1.59 g/mL (liquid, 35 °C) 12.25 kg/m³ (gas, 1 atm, 10 times air density) |
| Melting point | −115 °C (−175 °F; 158 K) |
| Boiling point | 28 °C (82 °F; 301 K) Heat of vaporization = 21 cal/g |
| Vapor pressure | 83.99 kPa (25 °C) |
| Viscosity | 0.652 mPa*s (25 °C) |
| Thermochemistry | |
Heat capacity (C) |
0.26 cal/(g • K) |
| Pharmacology | |
| V08DA03 (WHO) | |
| Hazards | |
| GHS labelling: | |
Pictograms |
 |
Signal word |
Warning |
Hazard statements |
H315, H319, H335 |
Precautionary statements |
P261, P264, P271, P280, P302+P352, P304+P340, P305+P351+P338, P312, P321, P332+P313, P337+P313, P362, P403+P233, P405, P501 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Perfluoropentane (PFP) or dodecafluoropentane; also known as Perflenapent (INN/USAN) is a fluorocarbon, the fluorinated analogue of pentane. It is a liquid that boils at slightly over room temperature.
It has several biomedical applications including: propellant for pressurized metered dose inhalers;[3] gas core in microbubble ultrasound contrast agents;[4] and occlusion therapy via the conversion of nanometer liquid droplets into micrometer sized gas microbubbles (acoustic droplet vaporization).[5]
References
- ↑ "Front Matter". Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. p. 33. doi:10.1039/9781849733069-FP001. ISBN 978-0-85404-182-4.
The prefix 'per-' is no longer recommended.
- ↑ "Perfluoropentane". Retrieved September 26, 2023.
- ↑ Rogueda, P. G. A. HPFP, a Model Propellant for pMDIs. Drug Dev. Ind. Phar. 2003, 29, 39
- ↑ Liu, Y., Miyoshi, H., and Nakamura, M. Encapsulated ultrasound microbubbles: Therapeutic application in drug/gene delivery. J. Controlled Release 2006, 114, 89− 99
- ↑ D. Bardin, T. D. Martz, P. S. Sheeran, R. Shih, P. A. Dayton, and A. P. Lee, “High-speed, clinical-scale microfluidic generation of stable phase-change droplets for gas embolotherapy,” Lab on a Chip, vol. 11, no. 23, p. 3990, 2011.
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.