Plasmodium cynomolgi
| Plasmodium cynomolgi | |
|---|---|
| Scientific classification | |
| Domain: | Eukaryota |
| Clade: | Diaphoretickes |
| Clade: | TSAR |
| Clade: | SAR |
| Clade: | Alveolata |
| Phylum: | Apicomplexa |
| Class: | Aconoidasida |
| Order: | Haemospororida |
| Family: | Plasmodiidae |
| Genus: | Plasmodium |
| Species: | P. cynomolgi |
| Binomial name | |
| Plasmodium cynomolgi (Mayer, 1907) | |
Plasmodium cynomolgi is an apicomplexan parasite that infects mosquitoes and Asian Old World monkeys. In recent years, a number of natural infections of humans have also been documented.[1][2][3][4][5][6][7][8] This species has been used as a model for human Plasmodium vivax because Plasmodium cynomolgi shares the same life cycle and some important biological features with P. vivax.[9]
Morphology

P. cynomolgi closely resembles the human parasite P. vivax throughout its life cycle. Similar to P. vivax, P. cynomolgi infection changes the red blood cell membrane structure, causing surface perturbations that appear as pink dots (called Schüffner's dots) when stained with Giemsa.[10]
Life cycle
The life cycle of P. cynomolgi resembles that of other Plasmodium species, particularly the related human parasite Plasmodium vivax.[11] Like other Plasmodium species, P. cynomolgi infects both an insect host and a vertebrate (generally Old World monkeys). The parasite is transmitted when the mosquito host takes a blood meal from the vertebrate host. During the feeding, motile parasites called sporozoites are injected from the mosquito salivary gland into the host tissue. These sporozoites move into the bloodstream and infect cells in the host liver, where they grow and divide over the course of approximately one week.[10] At this point, the parasitized liver cells rupture, releasing thousands of parasite daughter cells, called merozoites, which either move into the bloodstream to infect red blood cells, or remain in the liver to reinfect liver cells. Those that reinfect liver cells form a quiescent stage called a hypnozoite, which can remain dormant in the liver cell for months or years before reactivating.[10] The merozoites that enter the bloodstream infect red blood cells, where they grow and replicate. After approximately 48 hours, the infected red blood cell bursts, allowing the daughter merozoites to infect new red blood cells. This cycle can continue indefinitely. Occasionally, after infection of a red blood cell, the parasite develops into one of two distinct sexual forms called male and female gametocytes (also micro and macrogametocytes respectively). If a mosquito takes a blood meal containing a gametocyte of each sex, the two sexual stages merge and form a zygote.[10] The zygote develops into a motile stage called the ookinete which penetrates the wall of the mosquito gut and forms a stationary oocyst. The oocyst develops over about 11 days, then begins to release thousands of sporozoites into the mosquito's hemolymph. The sporozoites move through the hemolymph and infect the mosquito salivary glands, where they will again be injected into a mammalian host when the mosquito takes a blood meal.[10]
Distribution
P. cynomolgi is found throughout Southeast Asia where it naturally infects a variety of macaque monkeys, including Macaca cyclopis, Macaca fascicularis, Macaca mulatta, Macaca nemestrina, Macaca radiata, Macaca sinica, Trachypithecus cristatus, and Semnopithecus entellus.[10][12] The effect of infection on primate hosts has primarily been studied in rhesus monkeys, where P. cynomolgi generally causes mild and self-limiting illness.[10] Monkeys can suffer anemia and thrombocytopenia as well as occasional kidney inflammation, however all generally resolve without treatment.[10] The exception to this is in pregnant monkeys, where P. cynomolgi infection can be severe, resulting in death of the mother and fetus without antimalarial treatment.[10]
Taxonomy and evolution
P. cynomolgi is in the genus Plasmodium, which contains all Apicomplexan parasites that undergo asexual reproduction through schizogony and digest red blood cell hemoglobin to produce the crystalline pigment hemozoin. Within Plasmodium, P. cynomolgi is in the subgenus Plasmodium, containing all species of Plasmodium that infect primates (except for some that infect the Great Apes, which are in the subgenus Laverania).
Evolutionarily, P. cynomolgi is most closely related to the other Plasmodium species that infect monkeys, as well as P. vivax which infects humans. Evolutionary relationships among Plasmodium species have been inferred from ribosomal RNA sequencing, and are summarized in the cladogram below:[13]
|
Plasmodium subgenus Vinckeia (infects rodents) | |||||||||||||||||||
| |||||||||||||||||||
Research
P. cynomolgi is the second-most studied malaria parasite of non-human primates after Plasmodium knowlesi, primarily due to its similarity to the human parasite P. vivax.[11] In particular, P. cynomolgi is used as a model for hypnozoite biology as it (along with P. vivax) is one of the few Plasmodium species known to have this lifecycle stage.[11] P. cynomolgi can infect a variety of monkey species and can be transmitted by several common laboratory-grown mosquitoes.[14][11] Due to this, P. cynomolgi has been used in research on a broad variety of malaria topics including hypnozoite biology, host immune responses to infection, and to test the efficacy of antimalarial drugs and vaccines.[11]
Human infection
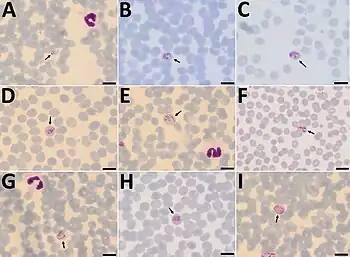
Infection of humans with P. cynomolgi was once thought to be exceedingly rare. However, documented cases of natural infection in humans have become more common in recent years, and initial misdiagnoses has led those researchers to theorize that other natural cases are being misidentified as P. vivax due to their morphological similarities.[12][16][1][2][3][4][5][6][7] Current evidence suggests that natural transmission is typically simian to human by a mosquito vector, but transmission of P. cynomolgi from human to human by a mosquito vector has also been shown in laboratory experiments.[12]
P. cynomolgi also infects a broad variety of Anopheles mosquitoes; the effect of infection on these mosquitoes is not known.[10]
History
P. cynomolgi was first observed in 1905 in the blood of the long-tailed macaque.[11]
References
- 1 2 Grignard, Lynn; Shah, Sonal; Chua, Tock H; William, Timothy; Drakeley, Chris J; Fornace, Kimberly M (2019-11-06). "Natural Human Infections With Plasmodium cynomolgi and Other Malaria Species in an Elimination Setting in Sabah, Malaysia". The Journal of Infectious Diseases. 220 (12): 1946–1949. doi:10.1093/infdis/jiz397. ISSN 0022-1899. PMC 6834065. PMID 31418017. Archived from the original on 2022-06-21. Retrieved 2023-02-01.
- 1 2 Imwong, Mallika; Madmanee, Wanassanan; Suwannasin, Kanokon; Kunasol, Chanon; Peto, Thomas J; Tripura, Rupam; von Seidlein, Lorenz; Nguon, Chea; Davoeung, Chan; Day, Nicholas P J; Dondorp, Arjen M (2019-02-15). "Asymptomatic Natural Human Infections With the Simian Malaria Parasites Plasmodium cynomolgi and Plasmodium knowlesi". The Journal of Infectious Diseases. 219 (5): 695–702. doi:10.1093/infdis/jiy519. ISSN 0022-1899. PMC 6376906. PMID 30295822. Archived from the original on 2022-08-02. Retrieved 2023-02-01.
- 1 2 Putaporntip, Chaturong; Kuamsab, Napaporn; Pattanawong, Urassaya; Yanmanee, Surasuk; Seethamchai, Sunee; Jongwutiwes, Somchai (February 2021). "Plasmodium cynomolgi Co-infections among Symptomatic Malaria Patients, Thailand". Emerging Infectious Diseases. 27 (2): 590–593. doi:10.3201/eid2702.191660. ISSN 1080-6040. PMC 7853550. PMID 33496236. Archived from the original on 2023-10-20. Retrieved 2023-02-01.
- 1 2 Raja, Thamayanthi Nada; Hu, Ting Huey; Kadir, Khamisah Abdul; Mohamad, Dayang Shuaisah Awang; Rosli, Nawal; Wong, Lolita Lin; Hii, King Ching; Simon Divis, Paul Cliff; Singh, Balbir (August 2020). "Naturally Acquired Human Plasmodium cynomolgi and P. knowlesi Infections, Malaysian Borneo". Emerging Infectious Diseases. 26 (8): 1801–1809. doi:10.3201/eid2608.200343. ISSN 1080-6040. PMC 7392409. PMID 32687020. Archived from the original on 2023-10-20. Retrieved 2023-02-01.
- 1 2 Singh, B.; Kadir, K.A.; Hu, T.H.; Raja, T.N.; Mohamad, D.S.; Lin, L.W.; Hii, K.C. (August 2018). "Naturally acquired human infections with the simian malaria parasite, Plasmodium cynomolgi, in Sarawak, Malaysian Borneo". International Journal of Infectious Diseases. 73: 68. doi:10.1016/j.ijid.2018.04.3581.
- 1 2 Ta, Thuy H; Hisam, Shamilah; Lanza, Marta; Jiram, Adela I; Ismail, NorParina; Rubio, José M (December 2014). "First case of a naturally acquired human infection with Plasmodium cynomolgi". Malaria Journal. 13 (1): 68. doi:10.1186/1475-2875-13-68. ISSN 1475-2875. PMC 3937822. PMID 24564912.
- 1 2 Yap, Nan Jiun; Hossain, Hanisah; Nada-Raja, Thamayanthi; Ngui, Romano; Muslim, Azdayanti; Hoh, Boon-Peng; Khaw, Loke Tim; Kadir, Khamisah Abdul; Simon Divis, Paul Cliff; Vythilingam, Indra; Singh, Balbir (August 2021). "Natural Human Infections with Plasmodium cynomolgi , P. inui , and 4 other Simian Malaria Parasites, Malaysia". Emerging Infectious Diseases. 27 (8): 2187–2191. doi:10.3201/eid2708.204502. ISSN 1080-6040. PMC 8314832. PMID 34287122. Archived from the original on 2023-10-20. Retrieved 2023-02-01.
- ↑ van Bergen, Kim; Stuitje, Toon; Akkers, Robert; Vermeer, Eric; Castel, Rob; Mank, Theo (December 2021). "Evaluation of a novel real-time PCR assay for the detection, identification and quantification of Plasmodium species causing malaria in humans". Malaria Journal. 20 (1): 314. doi:10.1186/s12936-021-03842-8. ISSN 1475-2875. PMC 8274047. PMID 34247622.
- ↑ "Plasmodium cynomolgi - Wellcome Trust Sanger Institute". www.sanger.ac.uk. Archived from the original on 2017-02-26. Retrieved 2016-12-01.
- 1 2 3 4 5 6 7 8 9 10 Galinski MR, Barnwell JW (2012). "Nonhuman primate models for human malaria research". Nonhuman Primates in Biomedical Research: Diseases. Elsevier. pp. 299–324.
- 1 2 3 4 5 6 Martinelli A, Culleton R (2018). "Non-human primate malaria parasites: out of the forest and into the laboratory". Parasitology. 145 (1): 41–54. doi:10.1017/S0031182016001335. PMID 27748213. S2CID 20884246.
- 1 2 3 Cormier LA (15 September 2011). "Ethics: Human Experimentation". The ten-thousand year fever: rethinking human and wild primate malarias. Routledge. pp. 127–130. ISBN 9781598744835.
- ↑ Leclerc MC, Hugot JP, Durand P, Renaud F (2004). "Evolutionary relationships between 15 Plasmodium species from New and Old World primates (including humans): an 18S rDNA cladistic analysis". Parasitology. 129 (6): 677–684. doi:10.1017/s0031182004006146. PMID 15648690. S2CID 14380959.
- ↑ Collins WE, Warren M, Galland GG (1999). "Studies on Infections with the Berok strain of Plasmodium cynomolgi in monkeys and mosquitoes". The Journal of Parasitology. 85 (2): 268–272. doi:10.2307/3285631. JSTOR 3285631. PMID 10219307.
- ↑ Hartmeyer, Gitte N.; Stensvold, Christen R.; Fabricius, Thilde; Marmolin, Ea S.; Hoegh, Silje V.; Nielsen, Henrik V.; Kemp, Michael; Vestergaard, Lasse S. "Plasmodium cynomolgi as Cause of Malaria in Tourist to Southeast Asia, 2018 - Volume 25, Number 10—October 2019 - Emerging Infectious Diseases journal - CDC". doi:10.3201/eid2510.190448.
{{cite journal}}: Cite journal requires|journal=(help) - ↑ Baird JK (2009). "Malaria zoonoses". Travel Medicine and Infectious Disease. 7 (5): 269–277. doi:10.1016/j.tmaid.2009.06.004. PMID 19747661.