Quinazolinone
 | |
| Names | |
|---|---|
| Preferred IUPAC name
Quinazolin-4(3H)-one | |
| Other names
4(3H)-Quinazolinone; 4(1H)-Quinazolinone; 3,4-Dihydroquinazolin-4-one; 4(3H)-Quinazolone; 4-Hydroxyquinazoline; 4-Oxo-3,4-dihydroquinazoline; 4-Oxoquinazoline; 4-Quinazolinol; 4-Quinazolinone; 4-Quinazolone | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
PubChem CID |
|
| UNII | |
InChI
| |
SMILES
| |
| Properties | |
Chemical formula |
C8H6N2O |
| Molar mass | 146.149 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
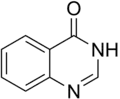
Quinazolinone is a heterocyclic chemical compound, a quinazoline with a carbonyl group in the C4N2 ring. Two isomers are possible: 2-quinazolinone and 4-quinazolinone, with the 4-isomer being the more common. These compounds are of interest in medicinal chemistry.[1]
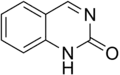 2-Quinazolinone
2-Quinazolinone 4-Quinazolinone
4-Quinazolinone
Synthesis
Common routes to quinazolines involve condensation of amides to anilines with ortho nitrile, carboxylic acids and amides.[2]
Quinazolinone drugs that function as hypnotic/sedatives usually contain a 4-quinazolinone core with a 2-substituted phenyl group at nitrogen atom 3.
See also
- Idelalisib (Zydelig)
- Methaqualone (Quaalude)
- Cloroqualone
- SL-164 (Dicloqualone)
- Diproqualone
- Etaqualone
- Mebroqualone
- Mecloqualone
- Methylmethaqualone
- Nitromethaqualone
References
- ↑ Jafari, E; et al. (2016), "Quinazolinone and quinazoline derivatives: recent structures with potent antimicrobial and cytotoxic activities", Res Pharm Sci, 11 (1): 1–14, PMC 4794932, PMID 27051427.
- ↑ Connolly, David J.; Cusack, Declan; O'Sullivan, Timothy P.; Guiry, Patrick J. (2005). "Synthesis of quinazolinones and quinazolines". Tetrahedron. 61 (43): 10153–10202. doi:10.1016/j.tet.2005.07.010.
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.