Acute radiation syndrome
| Acute radiation syndrome | |
|---|---|
| Other names: Radiation poisoning, radiation sickness, radiation toxicity | |
 | |
| Radiation causes cellular degradation by autophagy. | |
| Specialty | Critical care medicine |
| Symptoms | Early: Nausea, vomiting, loss of appetite[1] Later: Infections, bleeding, dehydration, confusion[1] |
| Complications | Cancer[2] |
| Usual onset | Within days[1] |
| Types | Bone marrow syndrome, gastrointestinal syndrome, neurovascular syndrome[1][3] |
| Causes | Large amounts of ionizing radiation over a short period of time[1] |
| Diagnostic method | Based on history of exposure and symptoms[4] |
| Treatment | Supportive care (blood transfusions, antibiotics, colony stimulating factors, stem cell transplant)[3] |
| Prognosis | Depends on the exposure dose[4] |
| Frequency | Rare[3] |
Acute radiation syndrome (ARS), also known as radiation sickness or radiation poisoning, is a collection of health effects due to exposure to high amounts of ionizing radiation over a short period of time.[1] Symptoms can begin within an hour and may last for several months.[1][3][5] Symptoms within the first few days typically include nausea, vomiting, and loss of appetite.[1] This is followed by a few hours or weeks with little symptoms which later develops into additional symptoms followed by either recovery or death.[1]
Acute radiation syndrome involves a total dose of greater than 0.7 Gy (70 rads), that generally occurs from a source outside the body within minutes.[1] Sources of such radiation can occur accidentally or intentionally.[6] They may involve nuclear reactors, cyclotrons, and certain devices used in cancer therapy.[4] It is generally divided into three types; bone marrow, gastrointestinal, and neurovascular syndrome, with bone marrow syndrome occurring at 0.7 to 10 Gy, and neurovascular syndrome occurring at doses that exceed 50 Gy.[1][3] The cells that are most affected are generally those that are rapidly dividing.[3] At high doses, this causes DNA damage that may be irreparable.[4] Diagnosis is based on a history of exposure and symptoms.[4] Repeated complete blood counts (CBCs) can indicate the severity of exposure.[1]
Treatment of acute radiation syndrome is generally supportive care.[3] This may include blood transfusions, antibiotics, colony-stimulating factors, or stem cell transplant.[3] If radioactive material remains on the skin or in the stomach it should be removed.[4] If radioiodine was breathed in or ingested, potassium iodide may be recommended.[4] Complications such as leukemia and other cancers among those who survive are managed as usual.[4] Short term outcomes depend on the exposure dose.[4]
ARS is generally rare.[3] A single event, however, can affect a relatively large number of people.[7] Notable cases occurred following the atomic bombing of Hiroshima and Nagasaki and the Chernobyl nuclear power plant disaster.[1] ARS differs from chronic radiation syndrome, which occurs following prolonged exposures to relatively low doses of radiation.[8][9] In 2023 the World Health Organization updated its list of medicines for radiological and nuclear emergencies.[10]
Signs and symptoms
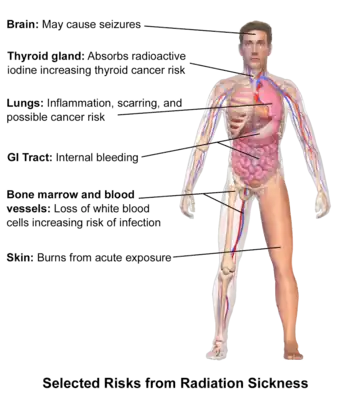
Classically acute radiation syndrome is divided into three main presentations: hematopoietic, gastrointestinal, and neurological/vascular. These syndromes may or may not be preceded by a prodrome.[3] The speed of onset of symptoms is related to radiation exposure, with greater doses resulting in a shorter delay in symptom onset.[3] These presentations presume whole-body exposure and many of them are markers that are not valid if the entire body has not been exposed. Each syndrome requires that the tissue showing the syndrome itself be exposed. [11][7] Some areas affected are:
- Hematopoietic. This syndrome is marked by a drop in the number of blood cells, called aplastic anemia. This may result in infections due to a low number of white blood cells, bleeding due to a lack of platelets, and anemia due to too few red blood cells in the circulation.[3] These changes can be detected by blood tests after receiving a whole-body acute dose as low as 0.3 Gray ,international system of unites, though they might never be felt by the patient if the dose is below 0.7 Gray. Conventional trauma and burns resulting from a bomb blast are complicated by the poor wound healing caused by hematopoietic syndrome, increasing mortality.[1][12][13]
- Gastrointestinal. This syndrome often follows absorbed doses of 6–30 grays (600–3,000 rad).[3] The signs and symptoms of this form of radiation injury include nausea, vomiting, loss of appetite, and abdominal pain.[14] Vomiting in this time-frame is a marker for whole body exposures that are in the fatal range above 4 grays (400 rad). Without exotic treatment such as bone marrow transplant, death with this dose is common.[3]
- Neurovascular. This syndrome typically occurs at absorbed doses greater than 30 grays (3,000 rad), though it may occur at 10 grays (1,000 rad).[3] It presents with neurological symptoms such as dizziness, headache, or decreased level of consciousness, occurring within minutes to a few hours, and with an absence of vomiting. It is invariably fatal.[3]
Early symptoms of ARS typically includes nausea and vomiting, headaches, fatigue, fever, and a short period of skin reddening.[3] These symptoms may occur at radiation doses as low as 0.35 grays (35 rad). These symptoms are common to many illnesses, and may not, by themselves, indicate acute radiation sickness.[3]
Dose effects
| Phase | Symptom | Whole-body absorbed dose (Gy) | ||||
|---|---|---|---|---|---|---|
| 1–2 Gy | 2–6 Gy | 6–8 Gy | 8–30 Gy | > 30 Gy | ||
| Immediate | Nausea and vomiting | 5–50% | 50–100% | 75–100% | 90–100% | 100% |
| Time of onset | 2–6 h | 1–2 h | 10–60 min | < 10 min | Minutes | |
| Duration | < 24 h | 24–48 h | < 48 h | < 48 h | N/A (patients die in < 48 h) | |
| Diarrhea | None | None to mild (< 10%) | Heavy (> 10%) | Heavy (> 95%) | Heavy (100%) | |
| Time of onset | — | 3–8 h | 1–3 h | < 1 h | < 1 h | |
| Headache | Slight | Mild to moderate (50%) | Moderate (80%) | Severe (80–90%) | Severe (100%) | |
| Time of onset | — | 4–24 h | 3–4 h | 1–2 h | < 1 h | |
| Fever | None | Moderate increase (10–100%) | Moderate to severe (100%) | Severe (100%) | Severe (100%) | |
| Time of onset | — | 1–3 h | < 1 h | < 1 h | < 1 h | |
| CNS function | No impairment | Cognitive impairment 6–20 h | Cognitive impairment > 24 h | Rapid incapacitation | Seizures, tremor, ataxia, lethargy | |
| Latent period | 28–31 days | 7–28 days | < 7 days | None | None | |
| Illness | Mild to moderate Leukopenia Fatigue Weakness |
Moderate to severe Leukopenia Purpura Hemorrhage Infections Alopecia after 3 Gy |
Severe leukopenia High fever Diarrhea Vomiting Dizziness and disorientation Hypotension Electrolyte disturbance |
Nausea Vomiting Severe diarrhea High fever Electrolyte disturbance Shock |
N/A (patients die in < 48h) | |
| Mortality | Without care | 0–5% | 5–95% | 95–100% | 100% | 100% |
| With care | 0–5% | 5–50% | 50–100% | 99–100% | 100% | |
| Death | 6–8 weeks | 4–6 weeks | 2–4 weeks | 2 days – 2 weeks | 1–2 days | |
| Table source[15] | ||||||
A person who happened to be less than a 1 mile (1.6 km) from the atomic bomb Little Boy's hypocenter at Hiroshima, Japan was found to absorb about 9.46 grays (Gy).[16][17][18][19]
The doses at the hypocenters of the Hiroshima and Nagasaki atomic bombings were 240 and 290 Gy, respectively.[20]
Skin changes
Cutaneous radiation syndrome (CRS) refers to the skin symptoms of radiation exposure.[1] Within a few hours after irradiation, a transient and inconsistent redness (associated with itching) can occur. Then, a latent phase may occur and last from a few days up to several weeks, when intense reddening, blistering, and ulceration of the irradiated site is visible. In most cases, healing occurs by regenerative means; however, very large skin doses can cause permanent hair loss, damaged sebaceous and sweat glands, atrophy, fibrosis (mostly keloids), decreased or increased skin pigmentation, and ulceration or necrosis of the exposed tissue.[1]
Notably, as seen at Chernobyl, when skin is irradiated with high energy beta particles, moist desquamation (peeling of skin) and similar early effects can heal, only to be followed by the collapse of the dermal vascular system after two months, resulting in the loss of the full thickness of the exposed skin.[21]
This effect had been demonstrated previously with pig skin using high energy beta sources at the Churchill Hospital Research Institute, in Oxford.[22]
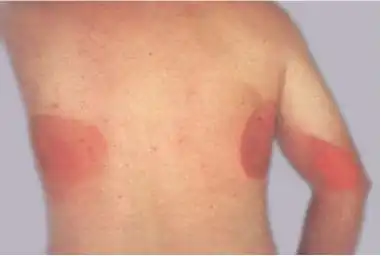 Ionizing radiation burn: large red patches of skin on the back and arm from multiple prolonged fluoroscopy procedures
Ionizing radiation burn: large red patches of skin on the back and arm from multiple prolonged fluoroscopy procedures.jpg.webp) Acute radiation dermatitis
Acute radiation dermatitis Skin appearance after the exposure to an atomic bomb.
Skin appearance after the exposure to an atomic bomb..jpg.webp) Chronic radiation dermatitis
Chronic radiation dermatitis
Cause
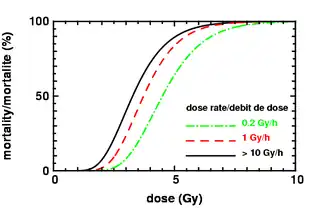
Radiation sickness is caused by exposure to a large dose of ionizing radiation (> ~0.1 Gy) over a short period of time (> ~0.1 Gy/h). Alpha and beta radiation have low penetrating power and are unlikely to affect vital internal organs from outside the body. Any type of ionizing radiation can cause burns, but alpha and beta radiation can only do so if radioactive contamination or nuclear fallout is deposited on the individual's skin or clothing. Gamma and neutron radiation can travel much further distances and penetrate the body easily, so whole-body irradiation generally causes ARS before skin effects are evident. Local gamma irradiation can cause skin effects without any sickness. In the early twentieth century, radiographers would commonly calibrate their machines by irradiating their own hands and measuring the time to onset of erythema.[27]
Accidental
Accidental exposure may be the result of a criticality or radiotherapy accident. There have been numerous criticality accidents dating back to atomic testing during World War II, while computer-controlled radiation therapy machines such as Therac-25 played a major part in radiotherapy accidents. The latter of the two is caused by the failure of equipment software used to monitor the radiational dose given. Human error has played a large part in accidental exposure incidents which include some of the criticality accidents, and larger scale events such as the Chernobyl disaster. Other events have to do with orphan sources in which radioactive material is unknowingly kept, sold, or stolen. The Goiânia accident is an example, where a forgotten radioactive source was taken from a hospital resulting in the death of 4 people from ARS.[28]
The Fukushima nuclear disaster was a 2011 nuclear accident at the Fukushima Daiichi Nuclear Power Plant in Ōkuma, Fukushima, Japan. The proximate cause of the nuclear disaster was the 2011 Tōhoku earthquake and tsunami natural disaster that occurred on 11 March 2011 and was the most powerful earthquake ever recorded in Japan. The earthquake triggered a powerful tsunami, with 13–14 meter high waves causing damage to the nuclear power plant. The result is the most severe nuclear accident since the Chernobyl disaster in 1986, classified as level seven on the International Nuclear Event Scale (INES), after initially being classified as level five,[29][30] joining Chernobyl as the only other accident to receive such classification.[31]
Exposure may also come from routine spaceflight and solar flares that result in radiation effects on earth in the form of solar storms. During spaceflight astronauts are exposed to both galactic cosmic radiation (GCR) and solar particle event (SPE) radiation. The exposure particularly occurs during flights beyond low Earth orbit (LEO). Evidence indicates past SPE radiation levels that would have been lethal for unprotected astronauts.[32] GCR levels that might lead to acute radiation poisoning are less well understood.[33]
Intentional
Intentional exposure is controversial as it involves the use of nuclear weapons, human experiments, or is given to a victim in an act of murder. The intentional atomic bombings of Hiroshima and Nagasaki resulted in tens of thousands of casualties; the survivors of these bombings are known today as Hibakusha. Nuclear weapons emit large amounts of thermal radiation as visible, infrared, and ultraviolet light, to which the atmosphere is largely transparent. This event is also known as "Flash", where radiant heat and light are bombarded into any given victim's exposed skin causing radiation burns.[34] Death is highly likely and radiation poisoning is almost certain if one is caught in the open with no terrain or building masking effects within a radius of 0–3 km from a 1 megaton airburst, and the 50% chance of death from the blast extends out to ~8 km from the same 1 megaton atmospheric explosion.[35]
Scientific testing on humans done without consent has been prohibited since 1997 in the United States. There is now a requirement for patients to give informed consent, and to be notified if experiments were classified.[36] Across the world, the Soviet nuclear program involved human experiments on a large scale which is still kept secret by the Russian government and the Rosatom agency.[37][38] Criminal activity has involved murder, and attempted murder carried out through abrupt victim contact with a radioactive substance such as polonium or plutonium.[39]
Pathophysiology
The most commonly used predictor of acute radiation symptoms is the whole-body absorbed dose. Several related quantities, such as the equivalent dose, effective dose, and committed dose, are used to gauge long-term stochastic biological effects such as cancer incidence, but they are not designed to evaluate acute radiation syndrome.[40] To help avoid confusion between these quantities, absorbed dose is measured in units of grays (in SI, unit symbol Gy) or rads (in CGS), while the others are measured in sieverts (in SI, unit symbol Sv) or rems (in CGS). 1 rad = 0.01 Gy and 1 rem = 0.01 Sv.[41]
In most of the acute exposure scenarios that lead to radiation sickness, the bulk of the radiation is external whole-body gamma, in which case the absorbed, equivalent and effective doses are all equal. There are exceptions, such as the Therac-25 accidents and the 1958 Cecil Kelley criticality accident, where the absorbed doses in Gy or rad are the only useful quantities, because of the targeted nature of the exposure to the body.[42][43][44]: 425
Radiotherapy treatments are typically prescribed in terms of the local absorbed dose, which might be 60 Gy or higher. The dose is fractionated, which allows for the normal tissues to undergo repair, allowing it to tolerate a higher dose than would otherwise be expected. The dose to the targeted tissue mass must be averaged over the entire body mass, most of which receives negligible radiation.[45][46][47]
DNA damage
Exposure to high doses of radiation can cause DNA damage, later creating serious and even lethal chromosomal aberrations if left unrepaired. Ionizing radiation can produce reactive oxygen species, and does direct damage to cells by causing localized ionization events. The former is very damaging to DNA, while the latter events create clusters of DNA damage.[48][49] This damage includes loss of nucleobases and breakage of the sugar-phosphate backbone that binds to the nucleobases. The DNA organization at the level of histones, nucleosomes, and chromatin also affects its susceptibility to radiation damage.[50] Clustered damage, defined as at least two lesions within a helical turn, is especially harmful.[49] While DNA damage happens frequently and naturally in the cell from endogenous sources, clustered damage is a unique effect of radiation exposure.[51] Clustered damage takes longer to repair than isolated breakages, and is less likely to be repaired at all.[52] Larger radiation doses are more prone to cause tighter clustering of damage, and closely localized damage is increasingly less likely to be repaired.[49]
Somatic mutations cannot be passed down from parent to offspring, but these mutations can propagate in cell lines within an organism. Radiation damage can also cause chromosome and chromatid aberrations, and their effect depends on what stage of the mitotic cycle the cell is currently in when the irradiation occurs. If the cell is in interphase, while it is still a single strand of chromatin, the damage will be replicated during the S1 phase of cell cycle, and there will be a break on both chromosome arms. Then the damage will be apparent in both daughter cells. If the irradiation occurs after replication, only one arm will bear the damage. This damage will only be apparent in one daughter cell. A damaged chromosome may cyclize, binding to another chromosome, or to itself.[53]
Diagnosis
Diagnosis is typically made based on a history of significant radiation exposure and suitable clinical findings.[3] An absolute lymphocyte count can give a rough estimate of radiation exposure.[3] Time from exposure to vomiting can also give estimates of exposure levels if they are less than 10 Gray (1000 rad).[3]
Prevention
A guiding principle of radiation safety is as low as reasonably achievable (ALARA).[54] This means try to avoid exposure as much as possible and includes the three components of time, distance, and shielding.[54]
Time
The longer that humans are subjected to radiation the larger the dose will be. The advice in the nuclear war manual entitled Nuclear War Survival Skills published by Cresson Kearny in the U.S. was that if one needed to leave the shelter then this should be done as rapidly as possible to minimize exposure.[55]
In chapter 12, he states that "[q]uickly putting or dumping wastes outside is not hazardous once fallout is no longer being deposited. For example, assume the shelter is in an area of heavy fallout and the dose rate outside is 400 roentgen (R) per hour, enough to give a potentially fatal dose in about an hour to a person exposed in the open. If a person needs to be exposed for only 10 seconds to dump a bucket, in this 1/360 of an hour he will receive a dose of only about 1 R. Under war conditions, an additional 1-R dose is of little concern." In peacetime, radiation workers are taught to work as quickly as possible when performing a task that exposes them to radiation.[55]
Shielding

Matter attenuates radiation in most cases, so placing any mass between humans and the source will reduce the radiation dose. though this is not always the case however. Care should be taken when constructing shielding for a specific purpose. For example, although high atomic number materials are very effective in shielding photons, using them to shield beta particles may cause higher radiation exposure due to the production of bremsstrahlung x-rays, and hence low atomic number materials are recommended. [56][7][57]
There are many types of shielding strategies that can be used to reduce the effects of radiation exposure. Internal contamination protective equipment such as respirators are used to prevent internal deposition as a result of inhalation and ingestion of radioactive material. Dermal protective equipment, which protects against external contamination, provides shielding to prevent radioactive material from being deposited on external structures.[58]
While these protective measures do provide a barrier from radioactive material deposition, they do not shield from externally penetrating gamma radiation. This leaves anyone exposed to penetrating gamma rays at high risk of Acute Radiation Syndrome.Naturally, shielding the entire body from high energy gamma radiation is optimal, but the required mass to provide adequate attenuation makes functional movement nearly impossible. In the event of a radiation catastrophe, medical and security personnel need mobile protection equipment in order to safely assist in containment, evacuation, and other public safety objectives.[59][60]
Research has been done exploring the feasibility of partial body shielding, a radiation protection strategy that provides adequate attenuation to only the most radio-sensitive organs and tissues inside the body. Irreversible stem cell damage in the bone marrow is the first life-threatening effect of intense radiation exposure and therefore one of the most important bodily elements to protect. Due to the regenerative property of hematopoietic stem cells, it is only necessary to protect enough bone marrow to repopulate the exposed areas of the body with the shielded supply.[61] This concept allows for the development of lightweight mobile radiation protection equipment, which provides adequate protection, deferring the onset of Acute Radiation Syndrome to much higher exposure doses. One example of such equipment is the 360 gamma, a radiation protection belt that applies selective shielding to protect the bone marrow stored in the pelvic area as well as other radio sensitive organs in the abdominal region without hindering functional mobility.[62][63][64]
Reduction of incorporation
Where radioactive contamination is present, a gas mask, dust mask, or good hygiene practices may offer protection, depending on the nature of the contaminant. Potassium iodide (KI) tablets can reduce the risk of cancer in some situations due to slower uptake of ambient radioiodine. Although this does not protect any organ other than the thyroid gland, their effectiveness is still highly dependent on the time of ingestion, which would protect the gland for the duration of a twenty-four-hour period. They do not prevent acute radiation syndrome as they provide no shielding from other environmental radionuclides.[65]
Fractionation of dose
If an intentional dose is broken up into a number of smaller doses, with time allowed for recovery between irradiations, the same total dose causes less cell death. Even without interruptions, a reduction in dose rate below 0.1 Gy/h also tends to reduce cell death.[40]
The human body contains many types of cells, for many short term radiation deaths, the loss of important types of cells that are constantly being regenerated causes death. The loss of cells forming blood cells (bone marrow), for example, is fatal.[66]
Management
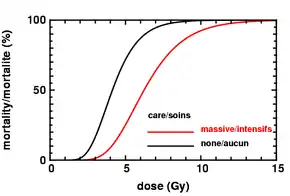
Treatment usually involves supportive care with possible symptomatic measures employed. The former involves the possible use of antibiotics, blood products, colony stimulating factors, and stem cell transplant.[3]
Antimicrobials
There is a direct relationship between the degree of the neutropenia that emerges after exposure to radiation and the increased risk of developing infection. Since there are no controlled studies of therapeutic intervention in humans, some recommendations are based on animal research.[67][68][69]
The treatment of established or suspected infection following exposure to radiation (characterized by neutropenia and fever) is similar to the one used for other febrile neutropenic patients. However, important differences between the two conditions exist. Individuals that develop neutropenia after exposure to radiation are also susceptible to irradiation damage in other tissues, such as the gastrointestinal tract, lungs and central nervous system. These patients may require therapeutic interventions not needed in other types of neutropenic patients. The response of irradiated animals to antimicrobial therapy can be unpredictable, as was evident in experimental studies where metronidazole[70] and pefloxacin[71] therapies were detrimental.
Antimicrobials that reduce the number of the strict anaerobic component of the gut flora (i.e., metronidazole) generally should not be given because they may enhance systemic infection by aerobic or facultative bacteria, thus facilitating mortality after irradiation.[72]
An empirical regimen of antimicrobials should be chosen based on the pattern of bacterial susceptibility and nosocomial infections in the affected area and medical center and the degree of neutropenia. Broad-spectrum empirical therapy (see below for choices) with high doses of one or more antibiotics should be initiated at the onset of fever. These antimicrobials should be directed at the eradication of Gram-negative aerobic bacilli (i.e., Enterobacteriace, Pseudomonas) that account for more than three quarters of the isolates causing sepsis. Because aerobic and facultative Gram-positive bacteria (mostly alpha-hemolytic streptococci) cause sepsis in about a quarter of the victims, coverage for these organisms may also be needed.[73]
A standardized management plan for people with neutropenia and fever should be devised. Empirical regimens contain antibiotics broadly active against Gram-negative aerobic bacteria (quinolones: i.e., ciprofloxacin, levofloxacin, a third- or fourth-generation cephalosporin with pseudomonal coverage: e.g., cefepime, ceftazidime, or an aminoglycoside: i.e. gentamicin, amikacin).[74]
Prognosis
The prognosis for ARS is dependent on the exposure dose, with anything above 8 Gy being almost always lethal even with medical care.[4][75] Radiation burns from lower-level exposures usually manifest after 2 months, while reactions from the burns occur months to years after radiation treatment.[76][77] Complications from ARS include an increased risk of developing radiation-induced cancer later in life. According to the linear no-threshold model, any exposure to ionizing radiation, even at doses too low to produce any symptoms of radiation sickness, can induce the cancer due to cellular and genetic damage. The probability of developing cancer is a linear function with respect to the effective radiation dose. Radiation cancer may occur following ionizing radiation exposure following a latent period averaging 20 to 40 years.[78][76]
History

Acute effects of ionizing radiation were first observed when Wilhelm Röntgen intentionally subjected his fingers to X-rays in 1895.[79] He published his observations concerning the burns that developed that eventually healed. Röntgen believed the free radical produced in air by X-rays from ozone was the cause, but other free radicals produced within the body are now understood to be more important.D. Walsh first established the symptoms of radiation sickness in 1897[80][81][82]
The atomic bombings of Hiroshima and Nagasaki resulted in high acute doses of radiation to a large number of Japanese people, allowing for greater insight into its symptoms and dangers. Red Cross Hospital Surgeon Terufumi Sasaki led intensive research into the syndrome in the weeks and months following the Hiroshima bombings. Dr Sasaki and his team were able to monitor the effects of radiation in patients of varying proximities to the blast itself, leading to the establishment of three recorded stages of the syndrome. Within 25–30 days of the explosion, the Red Cross surgeon noticed a sharp drop in white blood cell count and established this drop, along with symptoms of fever, as prognostic standards for Acute Radiation Syndrome.[83] Actress Midori Naka, who was present during the atomic bombing of Hiroshima, was the first incident of radiation poisoning to be extensively studied. Her death on 24 August 1945 was the first death ever to be officially certified as a result of acute radiation syndrome[84][85]
There are two major databases that track radiation accidents: The American ORISE REAC/TS and the European IRSN ACCIRAD. REAC/TS shows 417 accidents occurring between 1944 and 2000, causing about 3000 cases of acute radiation syndrome, of which 127 were fatal.[86] ACCIRAD lists 580 accidents with 180 ARS fatalities for an almost identical period,[87] (the two deliberate bombings are not included in either database, nor are any possible radiation-induced cancers from low doses.)
Notable cases
The following table includes only those known for their attempted survival with ARS. The "result" column represents the time of exposure to the time of death attributed to the short and long term effects attributed to initial exposure. As ARS is measured by a whole-body absorbed dose, the "exposure" column only includes units of Gray (Gy):
| Date | Name | Exposure (Gy) | Incident/accident | Result |
|---|---|---|---|---|
| August 21, 1945 | Harry Daghlian | 3.1 Gy[88] | Harry Daghlian criticality accident | Death in 25 days |
| May 21, 1946 | Louis Slotin | 11 Gy[89] | Slotin criticality accident | Death in 9 days |
| May 21, 1946 | Alvin C. Graves | 1.9 Gy[88] | Slotin criticality accident | Death in 19 years |
| December 30, 1958 | Cecil Kelley | 36 Gy[90] | Cecil Kelley criticality accident | Death in 38 hours |
| April 26, 1986 | Aleksandr Akimov | 15 Gy[91] | Chernobyl disaster | Death in 14 days |
Other animals
Many scientific experiments have been performed to study acute radiation syndrome in animals.[92] There is a simple guide for predicting survival / death in mammals, including humans, following the acute effects of inhaling radioactive particles.[93]
See also
- Nuclear safety and security
- 5-Androstenediol
- CBLB502
- Ex-Rad
- Orders of magnitude (radiation)
References
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 "A Fact Sheet for Physicians". CDC. CDC Radiation Emergencies Acute Radiation Syndrome. 22 April 2019. Archived from the original on 18 May 2019. Retrieved 17 May 2019.
- ↑ "Beir VII: Health Risks from Exposure to Low Levels of Ionizing Radiation" (PDF). The National Academy. Archived (PDF) from the original on 2020-03-07. Retrieved 2020-07-26.
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 Donnelly, EH; Nemhauser, JB; Smith, JM; Kazzi, ZN; Farfán, EB; Chang, AS; Naeem, SF (June 2010). "Acute radiation syndrome: assessment and management". Southern Medical Journal. 103 (6): 541–6. doi:10.1097/SMJ.0b013e3181ddd571. PMID 20710137. Archived from the original on 2019-06-26. Retrieved 2020-07-26.
- 1 2 3 4 5 6 7 8 9 10 "Radiation Sickness". National Organization for Rare Disorders. Archived from the original on 12 August 2019. Retrieved 6 June 2019.
- ↑ Xiao M, Whitnall MH (January 2009). "Pharmacological countermeasures for the acute radiation syndrome". Curr Mol Pharmacol. 2 (1): 122–133. doi:10.2174/1874467210902010122. PMID 20021452.
- ↑ Chao, NJ (April 2007). "Accidental or intentional exposure to ionizing radiation: biodosimetry and treatment options". Experimental Hematology. 35 (4 Suppl 1): 24–7. doi:10.1016/j.exphem.2007.01.008. PMID 17379083.
- 1 2 3 Acosta, Robert; Warrington, Steven J. (2022). "Radiation Syndrome". StatPearls. StatPearls Publishing. Archived from the original on 9 January 2020. Retrieved 12 October 2022.
- ↑ Akleyev, Alexander V. (2014). "chronic%20radiation%20syndrome"&pg=PA1 Chronic Radiation Syndrome. Springer Science & Business Media. p. 1. ISBN 9783642451171. Archived from the original on 2021-08-27. Retrieved 2022-03-15.
- ↑ Gusev, Igor; Guskova, Angelina; Mettler, Fred A. (2001). Medical Management of Radiation Accidents. CRC Press. p. 18. ISBN 9781420037197. Archived from the original on 2021-08-27. Retrieved 2020-07-26.
- ↑ "WHO updates critical medicines list for radiological and nuclear emergencies". www.who.int. Archived from the original on 27 January 2023. Retrieved 27 January 2023.
- ↑ Barabanova, A. V.; Bushmanov, A. J.; Kotenko, K. V. (1 January 2019). "Acute Radiation Sickness From Chernobyl☆". Reference Module in Earth Systems and Environmental Sciences. Elsevier. ISBN 978-0-12-409548-9. Archived from the original on 31 March 2022. Retrieved 10 October 2022.
- ↑ "Hematopoietic Subsyndrome of Acute Radiation Syndrome (ARS) - Radiation Emergency Medical Management". remm.hhs.gov. Archived from the original on 16 October 2022. Retrieved 14 October 2022.
- ↑ "The International System of Units (SI)" (PDF). Bureau International des Poids et Mesures (BIPM). Archived (PDF) from the original on 2017-08-14. Retrieved 2010-01-31.
- ↑ Christensen DM, Iddins CJ, Sugarman SL (February 2014). "Ionizing radiation injuries and illnesses". Emerg Med Clin North Am. 32 (1): 245–65. doi:10.1016/j.emc.2013.10.002. PMID 24275177.
- ↑ "Radiation Exposure and Contamination - Injuries; Poisoning - Merck Manuals Professional Edition". Merck Manuals Professional Edition. Retrieved 2017-09-06.
- ↑ Geggel, Laura (2018-05-01). "Human Bone Reveals How Much Radiation Hiroshima Bomb Released - And It's Staggering". livescience.com. Archived from the original on 2019-12-27. Retrieved 2019-12-27.
- ↑ Phillips, Kristine (2018-05-02). "A single jawbone has revealed just how much radiation Hiroshima bomb victims absorbed". Washington Post. Archived from the original on 2019-12-27. Retrieved 2019-12-27.
- ↑ Cullings, Harry M.; Fujita, Shoichiro; Funamoto, Sachiyo; Grant, Eric J.; Kerr, George D.; Preston, Dale L. (2006). "Dose Estimation for Atomic Bomb Survivor Studies: Its Evolution and Present Status". Radiation Research. Radiation Research Society. 166 (1): 219–254. Bibcode:2006RadR..166..219C. doi:10.1667/rr3546.1. ISSN 0033-7587. PMID 16808610.
- ↑ Ozasa, Kotaro; Grant, Eric J; Kodama, Kazunori (2018-04-05). "Japanese Legacy Cohorts: The Life Span Study Atomic Bomb Survivor Cohort and Survivors' Offspring". Journal of Epidemiology. Japan Epidemiological Association. 28 (4): 162–169. doi:10.2188/jea.je20170321. ISSN 0917-5040. PMC 5865006. PMID 29553058.
- ↑ Holdstock, Douglas (1995). Hiroshima and Nagasaki : retrospect and prospect. London Portland, Or: Frank Cass. p. 4. ISBN 978-1-135-20993-3. OCLC 872115191. Archived from the original on 2021-08-27. Retrieved 2020-07-26.
- ↑ The medical handling of skin lesions following high-level accidental irradiation, IAEA Advisory Group Meeting, September 1987 Paris.
- ↑ Wells J; et al. (1982), "Non-Uniform Irradiation of Skin: Criteria for limiting non-stochastic effects", Proceedings of the Third International Symposium of the Society for Radiological Protection, Advances in Theory and Practice, vol. 2, pp. 537–542, ISBN 978-0-9508123-0-4
- ↑ Zeitlin, C.; Hassler, D. M.; Cucinotta, F. A.; Ehresmann, B.; Wimmer-Schweingruber, R. F.; Brinza, D. E.; Kang, S.; Weigle, G.; Böttcher, S.; Böhm, E.; Burmeister, S.; Guo, J.; Köhler, J.; Martin, C.; Posner, A.; Rafkin, S.; Reitz, G. (1 May 2013). "Measurements of Energetic Particle Radiation in Transit to Mars on the Mars Science Laboratory". Science. 340: 1080–1084. doi:10.1126/science.1235989. ISSN 0036-8075. Retrieved 9 October 2022.
- ↑ Kerr, Richard (31 May 2013). "Radiation will make astronauts' trip to Mars even riskier". Science. 340 (6136): 1031. Bibcode:2013Sci...340.1031K. doi:10.1126/science.340.6136.1031. PMID 23723213.
- ↑ Chang, Kenneth (30 May 2013). "Data Point to Radiation Risk for Travelers to Mars". New York Times. Archived from the original on 31 May 2013. Retrieved 31 May 2013.
- ↑ Gelling, Cristy (June 29, 2013). "Mars trip would deliver big radiation dose; Curiosity instrument confirms expectation of major exposures". Science News. 183 (13): 8. doi:10.1002/scin.5591831304. Archived from the original on July 15, 2013. Retrieved July 8, 2013.
- ↑ Inkret, William C.; Meinhold, Charles B.; Taschner, John C. (1995). "A Brief History of Radiation Protection Standards" (PDF). Los Alamos Science (23): 116–123. Archived (PDF) from the original on 29 October 2012. Retrieved 12 November 2012.
- ↑ The Radiological accident in Goiânia (PDF). Vienna: International Atomic Energy Agency. 1988. ISBN 92-0-129088-8. Archived (PDF) from the original on 2016-03-12. Retrieved 2005-08-22.
- ↑ Brumfiel, Geoff (26 April 2011). "Nuclear agency faces reform calls". Nature. 472 (7344): 397–398. doi:10.1038/472397a. PMID 21528501. Archived from the original on 8 December 2021. Retrieved 15 October 2022.
- ↑ McCurry, Justin (12 April 2011). "Japan upgrades nuclear crisis to same level as Chernobyl". The Guardian. Archived from the original on 2 December 2020. Retrieved 14 December 2020.
- ↑ "Analysis: A month on, Japan nuclear crisis still scarring". International Business Times. 9 April 2011. Archived from the original on 15 August 2012. Retrieved 23 June 2021.
- ↑ "Superflares could kill unprotected astronauts". New Scientist. 21 March 2005. Archived from the original on 27 March 2015.
- ↑ National Research Council (U.S.). Ad Hoc Committee on the Solar System Radiation Environment and NASA's Vision for Space Exploration (2006). Space Radiation Hazards and the Vision for Space Exploration. National Academies Press. doi:10.17226/11760. ISBN 978-0-309-10264-3. Archived from the original on 2010-03-28.
- ↑ "Nuclear Bomb Effects". The Atomic Archive. solcomhouse.com. Archived from the original on 5 April 2014. Retrieved 12 September 2011.
- ↑ "Archive copy". Archived from the original on 2020-11-12. Retrieved 2020-07-26.
{{cite web}}: CS1 maint: archived copy as title (link) - ↑ "Human Radiation Experiments". www.atomicheritage.org. July 11, 2017. Archived from the original on December 30, 2019. Retrieved December 1, 2019.
- ↑ Федоров, Юрий. "Живущие в стеклянном доме". Радио Свобода (in Russian). Archived from the original on 2015-09-01. Retrieved 2015-08-31.
{{cite news}}: CS1 maint: unrecognized language (link) - ↑ "Slow Death In Kazakhstan's Land Of Nuclear Tests". RadioFreeEurope/RadioLiberty. 2011-08-29. Archived from the original on 2016-09-20. Retrieved 2015-08-31.
- ↑ Neuman, Scott (22 September 2021). "Russia Fatally Poisoned A Prominent Defector In London, A Court Concludes". NPR. Archived from the original on 1 October 2022. Retrieved 12 October 2022.
- 1 2 Icrp (2007). "The 2007 Recommendations of the International Commission on Radiological Protection". Annals of the ICRP. ICRP publication 103. 37 (2–4). ISBN 978-0-7020-3048-2. Archived from the original on 16 November 2012. Retrieved 17 May 2012.
- ↑ The Effects of Nuclear Weapons (Revised ed.). US Department of Defense. 1962. p. 579.
- ↑ Accidental Radiation Excursion at the Y-12 Plant (PDF) (Report). 1958. Archived (PDF) from the original on 2022-01-05. Retrieved 2022-10-10. Y-1234.
- ↑ Miner, William N.; Schonfeld, Fred W. (1968). "Plutonium". In Clifford A. Hampel (ed.). The Encyclopedia of the Chemical Elements. New York: Reinhold Book Corporation. pp. 540–546. LCCN 68-29938.
- ↑ Baase, Sara (5 August 2012). "8.2 Case Study: The Therac-25". A Gift of Fire: Social, Legal, and Ethical Issues for Computing Technology (application/ld+json) (4th ed.). Pearson Prentice Hall. pp. 425–430. ISBN 978-0132492676. LCCN 2012020988. OCLC 840390999. OL 25355635M – via Internet Archive.
- ↑ "Radiotherapy | Doctor". patient.info. Archived from the original on 9 July 2017. Retrieved 11 October 2022.
- ↑ "Radiation Therapy". medlineplus.gov. Archived from the original on 22 September 2022. Retrieved 11 October 2022.
- ↑ "Radiotherapy dose fractionation, third edition | The Royal College of Radiologists". www.rcr.ac.uk. Archived from the original on 2 August 2022. Retrieved 18 October 2022.
- ↑ Yu, Y.; Cui, Y.; Niedernhofer, L.; Wang, Y. (2016). "Occurrence, biological consequences and human health relevance of oxidative stress-induced DNA damage". Chemical Research in Toxicology. 29 (12): 2008–2039. doi:10.1021/acs.chemrestox.6b00265. PMC 5614522. PMID 27989142.
- 1 2 3 Eccles, L.; O'Neill, P.; Lomax, M. (2011). "Delayed repair of radiation induced DNA damage: Friend or foe?". Mutation Research. 711 (1–2): 134–141. doi:10.1016/j.mrfmmm.2010.11.003. PMC 3112496. PMID 21130102.
- ↑ Lavelle, C.; Foray, N. (2014). "Chromatin structure and radiation-induced DNA damage: From structural biology to radiobiology". International Journal of Biochemistry & Cell Biology. 49: 84–97. doi:10.1016/j.biocel.2014.01.012. PMID 24486235.
- ↑ Goodhead, D. (1994). "Initial events in the cellular effects of ionizing radiations: Clustered damage in DNA". International Journal of Radiation Biology. 65 (1): 7–17. doi:10.1080/09553009414550021. PMID 7905912.
- ↑ Georgakilas, A.; Bennett, P.; Wilson, D.; Sutherland, B. (2004). "Processing of bistranded abasic DNA clusters in gamma-irradiated human hematopoietic cells". Nucleic Acids Research. 32 (18): 5609–5620. doi:10.1093/nar/gkh871. PMC 524283. PMID 15494449.
- ↑ Hall, E.; Giaccia, A. (2006). Radiobiology for the Radiobiologist (6th ed.). Lippincott Williams & Wilkins.
- 1 2 "Radiation Safety". Centers for Disease Control and Prevention. 7 December 2015. Archived from the original on 7 May 2020. Retrieved 23 April 2020.
- 1 2 Kearny, Cresson H. (1988). Nuclear War Survival Skills. Oregon Institute of Science and Medicine. ISBN 978-0-942487-01-5. Archived from the original on 17 October 2017.
- ↑ "IAEA Nuclear Safety and Security Glossary". www.iaea.org. 14 November 2017. Archived from the original on 13 October 2022. Retrieved 12 October 2022.
- ↑ Barthe, Nicole; Maîtrejean, Serge; Cardona, Ana (1 January 2012). "Chapter 19 - High-Resolution Beta Imaging". Handbook of Radioactivity Analysis (Third Edition). Academic Press. pp. 1209–1242. ISBN 978-0-12-384873-4. Archived from the original on 3 July 2019. Retrieved 17 October 2022.
- ↑ "Personal Protective Equipment (PPE) in a Radiation Emergency". www.remm.nlm.gov. Radiation Emergency Medical Management. Archived from the original on 21 June 2018. Retrieved 26 June 2018.
- ↑ Budošová, Darina; Horváthová, Martina; Bárdyová, Zuzana; Balázs, Tibor (22 August 2022). "CURRENT TRENDS OF RADIATION PROTECTION EQUIPMENT IN INTERVENTIONAL RADIOLOGY". Radiation Protection Dosimetry. 198 (9–11): 554–559. doi:10.1093/rpd/ncac098. Archived from the original on 14 October 2022. Retrieved 13 October 2022.
- ↑ Frane, Nicholas; Bitterman, Adam (2022). "Radiation Safety and Protection". StatPearls. StatPearls Publishing. Archived from the original on 23 January 2022. Retrieved 13 October 2022.
- ↑ Waterman, Gideon; Kase, Kenneth; Orion, Itzhak; Broisman, Andrey; Milstein, Oren (September 2017). "Selective Shielding of Bone Marrow". Health Physics. 113 (3): 195–208. doi:10.1097/hp.0000000000000688. ISSN 0017-9078. PMID 28749810.
- ↑ Waterman, Gideon; Kase, Kenneth; Orion, Itzhak; Broisman, Andrey; Milstein, Oren (September 2017). "Selective Shielding of Bone Marrow: An Approach to Protecting Humans from External Gamma Radiation". Health Physics. 113 (3): 195–208. doi:10.1097/HP.0000000000000688. ISSN 1538-5159. Archived from the original on 5 March 2022. Retrieved 9 October 2022.
- ↑ "Occupational Radiation Protection in Severe Accident Management". Nuclear Energy Agency (NEA). Archived from the original on 8 December 2021. Retrieved 9 October 2022.
- ↑ "The Belt That Protects Against Gamma Radiation". Popular Mechanics. 27 June 2018. Archived from the original on 6 December 2021. Retrieved 16 October 2022.
- ↑ "Radiation and its Health Effects". Nuclear Regulatory Commission. Archived from the original on 14 October 2013. Retrieved 19 November 2013.
- ↑ Green, Danielle E.; Rubin, Clinton T. (2014). "Consequences of irradiation on bone and marrow phenotypes, and its relation to disruption of hematopoietic precursors". Bone. 0: 87–94. doi:10.1016/j.bone.2014.02.018. ISSN 8756-3282. Archived from the original on 17 October 2022. Retrieved 17 October 2022.
- ↑ "Neutropenia and Risk for Infection". Centers for Disease Control and Prevention. 2 December 2021. Archived from the original on 2 August 2022. Retrieved 14 October 2022.
- ↑ "Radiation Sickness". NORD (National Organization for Rare Disorders). Archived from the original on 12 August 2019. Retrieved 16 October 2022.
- ↑ Mac Manus, Michael; Lamborn, Kathleen; Khan, Waqqar; Varghese, Anna; Graef, Lorin; Knox, Susan (1 April 1997). "Radiotherapy-Associated Neutropenia and Thrombocytopenia: Analysis of Risk Factors and Development of a Predictive Model". Blood. 89 (7): 2303–2310. doi:10.1182/blood.V89.7.2303. Archived from the original on 22 October 2022. Retrieved 22 October 2022.
- ↑ Brook, I.; Ledney, G.D. (1994). "Effect of antimicrobial therapy on the gastrointestinal bacterial flora, infection and mortality in mice exposed to different doses of irradiation". Journal of Antimicrobial Chemotherapy. 33 (1): 63–74. doi:10.1093/jac/33.1.63. ISSN 1460-2091. PMID 8157575. Archived from the original on 2020-09-25. Retrieved 2020-07-26.
- ↑ Patchen ML, Brook I, Elliott TB, Jackson WE (1993). "Adverse effects of pefloxacin in irradiated C3H/HeN mice: correction with glucan therapy". Antimicrobial Agents and Chemotherapy. 37 (9): 1882–1889. doi:10.1128/AAC.37.9.1882. ISSN 0066-4804. PMC 188087. PMID 8239601.
- ↑ Brook I, Walker RI, MacVittie TJ (1988). "Effect of antimicrobial therapy on the bowel flora and bacterial infection in irradiated mice". International Journal of Radiation Biology. 53 (5): 709–718. doi:10.1080/09553008814551081. ISSN 1362-3095. PMID 3283066. Archived from the original on 2020-09-23. Retrieved 2020-07-26.
- ↑ Brook I, Ledney D (1992). "Quinolone therapy in the management of infection after irradiation". Crit Rev Microbiol. 18 (4): 18235–18246. doi:10.3109/10408419209113516. PMID 1524673.
- ↑ Brook I, Elliot TB, Ledney GD, Shomaker MO, Knudson GB (2004). "Management of postirradiation infection: lessons learned from animal models". Military Medicine. 169 (3): 194–197. doi:10.7205/MILMED.169.3.194. ISSN 0026-4075. PMID 15080238.
- ↑ "Time Phases of Acute Radiation Syndrome (ARS) - Dose >8 Gy". Radiation Emergency Medical Management. Archived from the original on June 28, 2019. Retrieved December 1, 2019.
- 1 2 James, W.; Berger, T.; Elston, D. (2005). Andrews' Diseases of the Skin: Clinical Dermatology (10th ed.). Saunders. ISBN 0-7216-2921-0.
- ↑ Wagner, L. K.; McNeese, M. D.; Marx, M. V.; Siegel, E. L. (1999). "Severe skin reactions from interventional fluoroscopy: case report and review of the literature". Radiology. 213 (3): 773–776. doi:10.1148/radiology.213.3.r99dc16773. PMID 10580952.
- ↑ Gawkrodger, D. J. (2004). "Occupational skin cancers" (PDF). Occupational Medicine. London. 54 (7): 458–63. doi:10.1093/occmed/kqh098. PMID 15486177. Archived (PDF) from the original on 2016-08-04. Retrieved 2020-07-26.
- ↑ "X-Rays | Science Mission Directorate". science.nasa.gov. Archived from the original on 14 May 2017. Retrieved 16 October 2022.
- ↑ Sen, Mrittika; Honavar, Santosh G (October 2021). "Wilhelm Conrad Röntgen: Finding X". Indian Journal of Ophthalmology. 69 (10): 2570–2572. doi:10.4103/ijo.IJO_2321_21. ISSN 0301-4738. Archived from the original on 19 October 2022. Retrieved 19 October 2022.
- ↑ Trapp, Jamie V.; Kron, Tomas (13 March 2008). An Introduction to Radiation Protection in Medicine. CRC Press. p. 1. ISBN 978-1-58488-965-6. Archived from the original on 22 October 2022. Retrieved 22 October 2022.
- ↑ Sonntag, Clemens von; Gunten, Urs von (31 August 2012). Chemistry of Ozone in Water and Wastewater Treatment. IWA Publishing. p. 2. ISBN 978-1-84339-313-9. Archived from the original on 24 October 2022. Retrieved 24 October 2022.
- ↑ Carmichael, Ann G. (1991). Medicine: A Treasury of Art and Literature. New York: Harkavy Publishing Service. p. 376. ISBN 978-0-88363-991-7.
- ↑ Langley, Paul J. (2008). Sacred Ground. State Library of South Australia, p. 2-3 Archived 2009-10-06 at the Wayback Machine
- ↑ Marks, Toshiko & Nish, Ian Hill & Prichard John B. (1989). Japan and the Second World War. "International Studies", Vol. 197. Suntory Toyota International Centre for Economics and Related Disciplines, London School of Economics and Political Science, p. 6.
- ↑ Turai, István; Veress, Katalin (2001). "Radiation Accidents: Occurrence, Types, Consequences, Medical Management, and the Lessons to be Learned". Central European Journal of Occupational and Environmental Medicine. 7 (1): 3–14. Archived from the original on 15 May 2013. Retrieved 1 June 2012.
- ↑ Chambrette, V.; Hardy, S.; Nenot, J.C. (2001). "Les accidents d'irradiation: Mise en place d'une base de données "ACCIRAD" à I'IPSN" (PDF). Radioprotection. 36 (4): 477–510. doi:10.1051/radiopro:2001105. Archived (PDF) from the original on 4 March 2016. Retrieved 13 June 2012.
- 1 2 Hempelman, Louis Henry; Lushbaugh, Clarence C.; Voelz, George L. (October 19, 1979). What Has Happened to the Survivors of the Early Los Alamos Nuclear Accidents? (PDF). Conference for Radiation Accident Preparedness. Oak Ridge: Los Alamos Scientific Laboratory. LA-UR-79-2802. Archived (PDF) from the original on September 12, 2014. Retrieved January 5, 2013. Patient numbers in this document have been identified as: 1 - Daghlian, 2 - Hemmerly, 3 - Slotin, 4 - Graves, 5 - Kline, 6 - Young, 7 - Cleary, 8 - Cieleski, 9 - Schreiber, 10 - Perlman
- ↑ Lawrence, James N. P. (6 October 1978). "Internal Memorandum on Los Alamos Criticality Accidents, 1945–1946, Personnel Exposures". Los Alamos Scientific Laboratory. H-l-78.
{{cite journal}}: Cite journal requires|journal=(help) - ↑ Harold, Catherine, ed. (2009). Professional guide to diseases (9th ed.). Philadelphia, PA: Lippincott Williams & Wilkins. ISBN 978-0-7817-7899-2. OCLC 475981026.
- ↑ Serhii Plokhii (2018). Chernobyl: the history of a nuclear catastrophe. Basic Books. ISBN 9781541617087. Archived from the original on 2021-08-27. Retrieved 2020-07-26.
- ↑ MacVittie, Thomas J.; Farese, Ann M.; Jackson, William III (November 2015). "The Hematopoietic Syndrome of the Acute Radiation Syndrome in Rhesus Macaques: A Systematic Review of the Lethal Dose Response Relationship". Health Physics. 109 (5): 342–366. doi:10.1097/HP.0000000000000352. ISSN 0017-9078. Archived from the original on 2 August 2022. Retrieved 15 October 2022.
- ↑ Wells, J. (1976). "A guide to the prognosis for survival in mammals following the acute effects of inhaled radioactive particles". Journal of the Institute of Nuclear Engineers. 17 (5): 126–131. ISSN 0368-2595.
- This article incorporates public domain material from websites or documents of the U.S. Armed Forces Radiobiology Research Institute and the U.S. Centers for Disease Control and Prevention
Further reading
WHO
- "National stockpiles for radiological and nuclear emergencies: policy advice". www.who.int. World Health Organization. Archived from the original on 27 January 2023. Retrieved 27 January 2023.
National/other
- "Fact sheet on Acute Radiation Syndrome". U.S. Centers for Disease Control and Prevention. Archived from the original on 16 July 2006. Retrieved 22 July 2006.
- "The criticality accident in Sarov" (PDF). International Atomic Energy Agency. 2001. Archived (PDF) from the original on 2012-02-04. Retrieved 2020-07-26. – A well documented account of the biological effects of a criticality accident.
- "Acute Radiation Syndrome (ARS) - Radiation Emergency Medical Management". remm.hhs.gov. Archived from the original on 15 August 2022. Retrieved 10 October 2022.
- "Armed Forces Radiobiology Research Institute". Archived from the original on 2015-03-03. Retrieved 2020-07-26.
External links
| Classification | |
|---|---|
| External resources |