Radioimmunotherapy
| Radioimmunotherapy | |
|---|---|
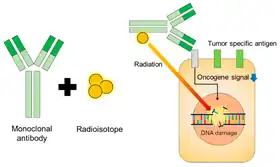 Schematic of radioimmunotherapy (RIT) | |
| Other names | RIT |
| ICD-9-CM | 92.28 |
| MeSH | D016499 |
Radioimmunotherapy (RIT) uses an antibody labeled with a radionuclide to deliver cytotoxic radiation to a target cell.[1] It is a form of unsealed source radiotherapy. In cancer therapy, an antibody with specificity for a tumor-associated antigen is used to deliver a lethal dose of radiation to the tumor cells. The ability for the antibody to specifically bind to a tumor-associated antigen increases the dose delivered to the tumor cells while decreasing the dose to normal tissues. By its nature, RIT requires a tumor cell to express an antigen that is unique to the neoplasm or is not accessible in normal cells.
History of available agents
| Name | Description | FDA status | EMA status |
| Ibritumomab tiuxetan (Zevalin) | monoclonal antibody anti-CD20 conjugated to a molecule that chelates Yttrium-90. | Approved (2002)[2][3] | Authorised (2004)[4] |
| Iodine (131I) tositumomab (Bexxar) | links a molecule containing Iodine-131 to an anti-CD20 monoclonal antibody | Approved (2003)[5] Withdrawn (2014)[6] | Orphan drug (2003) Withdrawn (2015)[7] |
| Lutetium (177Lu) lilotomab satetraxetan (Betalutin) | combination of lutetium-177 and an anti-CD37 monoclonal antibody | Fast track (2020)[8] | Orphan drug (2020)[9] |
131I tositumomab and 90Y ibritumomab tiuxetan were the first agents of radioimmunotherapy, and they were approved for the treatment of refractory non-Hodgkin's lymphoma. This means they are used in patients whose lymphoma is refractory to conventional chemotherapy and the monoclonal antibody rituximab.
Agents in clinical development
A set of radioimmunotherapy drugs that rely upon an alpha-emitting isotope (e.g., bismuth-213 or, preferably, actinium-225), rather than a beta emitter, as the killing source of radiation is being developed. Several phase II clinical trials for the treatment of acute myeloid leukemia have been carried out using alpha-emitting RITs.[10][11]
90Y-FF-21101 is a monoclonal antibody against P-cadherin radiolabeled with yttrium-90.[12] It is one of several RIT treatments under investigation intending to treat solid tumors.[13] A phase I clinical trial began in 2015.[14]
Other applications (non-approved indications)
Other types of cancer for which RIT has therapeutic potential include prostate cancer,[15] metastatic melanoma,[16] ovarian cancer,[17] neoplastic meningitis,[17] leukemia,[18] high-grade brain glioma,[19] and metastatic colorectal cancer.[20]
References
- ↑ Milenic, Diane E.; Brady, Erik D.; Brechbiel, Martin W. (June 2004). "Antibody-targeted radiation cancer therapy". Nature Reviews Drug Discovery. 3 (6): 488–499. doi:10.1038/nrd1413. PMID 15173838. S2CID 22166498.
- ↑ FIbritumomab Tiuxetan (Zevalin™) Radioimmunotherapy of Non-Hodgkin’s Lymphoma
- ↑ Rao AV, Akabani G, Rizzieri DA. Radioimmunotherapy for Non-Hodgkin's Lymphoma. Clin Med Res. 2005 Aug;3(3):157-65.
- ↑ "Zevalin". European Medicines Agency. Retrieved 8 November 2020.
- ↑ Tositumomab and Iodine I 131 Tositumomab - Product Approval Information - Licensing Action
- ↑ "Why Good Drugs Sometimes Fail: The Bexxar Story". 2013-08-26.
- ↑ "EU/3/03/136". European Medicines Agency. Retrieved 8 November 2020.
- ↑ "FDA grants fast track status to Betalutin for marginal zone lymphoma". Healio. 29 June 2020.
- ↑ "EU/3/20/2280". European Medicines Agency. Retrieved 8 November 2020.
- ↑ Bodet-Milin, Caroline; Kraeber-Bodéré, Françoise; Eugène, Thomas; Guérard, François; Gaschet, Joëlle; Bailly, Clément; Mougin, Marie; Bourgeois, Mickaël; Faivre-Chauvet, Alain; Chérel, Michel; Chevallier, Patrice (March 2016). "Radioimmunotherapy for Treatment of Acute Leukemia". Seminars in Nuclear Medicine. 46 (2): 135–146. doi:10.1053/j.semnuclmed.2015.10.007. PMID 26897718.
- ↑ Pandit-Taskar, Neeta (December 2019). "Targeted Radioimmunotherapy and Theranostics with Alpha Emitters". Journal of Medical Imaging and Radiation Sciences. 50 (4): S41–S44. doi:10.1016/j.jmir.2019.07.006. PMID 31451417.
- ↑ Subbiah, Vivek; Erwin, William; Mawlawi, Osama; McCoy, Asa; Wages, David; Wheeler, Catherine; Gonzalez-Lepera, Carlos; Liu, Holly; Macapinlac, Homer; Meric-Bernstam, Funda; Hong, David S.; Pant, Shubham; Le, Dao; Santos, Elmer; Gonzalez, Jose; Roszik, Jason; Suzuki, Takeaki; Subach, Ruth Ann; Madden, Timothy; Johansen, Mary; Nomura, Fumiko; Satoh, Hirokazu; Matsuura, Tadashi; Kajita, Masamichi; Nakamura, Eri; Funase, Yuichi; Matsushima, Satoshi; Ravizzini, Gregory (18 August 2020). "Phase I Study of P-cadherin–targeted Radioimmunotherapy with 90 Y-FF-21101 Monoclonal Antibody in Solid Tumors". Clinical Cancer Research. 26 (22): 1078–0432.CCR–20-0037. doi:10.1158/1078-0432.CCR-20-0037. PMID 32816889.
- ↑ Zaheer, Javeria; Kim, Hyeongi; Lee, Yong-Jin; Kim, Jin Su; Lim, Sang Moo (8 November 2019). "Combination Radioimmunotherapy Strategies for Solid Tumors". International Journal of Molecular Sciences. 20 (22): 5579. doi:10.3390/ijms20225579. PMC 6888084. PMID 31717302.
- ↑ A Phase 1 Dose-escalation Study of Radio- Labeled Antibody, FF-21101(90Y) for the Treatment of Advanced Cancer
- ↑ Smith-Jones PM. Radioimmunotherapy of prostate cancer. Q J Nucl Med Mol Imaging. 2004 Dec;48(4):297-304.
- ↑ Dadachova E, Nosanchuk JD, Shi L, Schweitzer AD, Frenkel A, Nosanchuk JS, and Casadevall A. Dead cells in melanoma tumors provide abundant antigen for targeted delivery of ionizing radiation by a monoclonal antibody to melanin. Proc Natl Acad Sci USA 2004;101: 14865-70.
- 1 2 Zalutsky MR, Pozzi OR. Radioimmunotherapy with alpha-particle emitting radionuclides. Q J Nucl Med Mol Imaging. 2004 Dec;48(4):289-96.
- ↑ Burke JM, Jurcic JG. Radioimmunotherapy of leukemia. Adv Pharmacol. 2004;51:185-208.
- ↑ Quang TS, Brady LW. Radioimmunotherapy as a novel treatment regimen: 125I-labeled monoclonal antibody 425 in the treatment of high-grade brain gliomas. Int J Radiat Oncol Biol Phys. 2004 Mar 1;58(3):972-5.
- ↑ Wong JY, Shibata S, Williams LE, Kwok CS, Liu A, Chu DZ, Yamauchi DM, Wilczynski S, Ikle DN, Wu AM, Yazaki PJ, Shively JE, Doroshow JH, Raubitschek AA. A Phase I trial of 90Y-anti-carcinoembryonic antigen chimeric T84.66 radioimmunotherapy with 5-fluorouracil in patients with metastatic colorectal cancer. Clin Cancer Res. 2003 Dec 1;9(16 Pt 1):5842-52.
External links
- Radioimmunotherapy at the US National Library of Medicine Medical Subject Headings (MeSH)
- Radioimmunotherapy.org