Selinexor
 | |
| Names | |
|---|---|
| Trade names | Xpovio, Nexpovio |
| Other names | KPT-330 |
IUPAC name
| |
| Clinical data | |
| Drug class | Inhibitor of nuclear export[1] |
| Main uses | Multiple myeloma[2] |
| Side effects | Nausea, weight loss, diarrhea, tiredness, low platelets, low red blood cells, low white blood cells, low sodium[1] |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Pregnancy category |
|
| Routes of use | By mouth |
| Typical dose | 80 mg[2] |
| External links | |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a619044 |
| Legal | |
| License data | |
| Legal status | |
| Pharmacokinetics | |
| Protein binding | 95% |
| Metabolism | Liver oxidation, glucuronidation, and conjugation, by CYP3A4, UGT and GST |
| Elimination half-life | 6–8 hours |
| Chemical and physical data | |
| Formula | C17H11F6N7O |
| Molar mass | 443.313 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
Selinexor sold under the brand name Xpovio among others, is a medication used to treat multiple myeloma.[2] It is used in cases were at least 4 other treatments have been found ineffective.[2] It is taken by mouth together with dexamethasone.[2][1]
Common side effects include nausea, weight loss, diarrhea, tiredness, low platelets, low red blood cells, low white blood cells, and low sodium.[1] Other side effects may include syncope.[2] Use during pregnancy may harm the baby.[2] It is a inhibitor of nuclear export and works by blocking the action of exportin 1.[1]
Selinexor was approved for medical use in the United States in 2019 and Europe in 2021.[2][1] In the United States it costs about 24,000 USD every 4 weeks.[4] While approved in the United Kingdom, it is not available there as of 2021.[5]
Medical uses
Selinexor is approved in combination with bortezomib and dexamethasone for the treatment of adults with multiple myeloma who have received at least one prior therapy.[6] Selinexor is also approved for use in combination with the steroid dexamethasone in people with relapsed or refractory multiple myeloma who have received at least four prior therapies and whose disease is refractory to at least two proteosome inhibitors, at least two immunomodulatory agents, and an anti-CD38 monoclonal antibody (so-called "quad-refractory" or "penta-refractory" myeloma),[7] for whom no other treatment options are available.[8][9] It is the first drug to be approved for this indication.[10]
In June 2020, the U.S. Food and Drug Administration (FDA) approved an additional indication for selinexor to treat adults with relapsed or refractory diffuse large B-cell lymphoma (DLBCL), not otherwise specified, including DLBCL arising from follicular lymphoma, after at least two lines of systemic therapy.[11]
In the European Union, selinexor is indicated in combination with dexamethasone for the treatment of multiple myeloma in adults who have received at least four prior therapies and whose disease is refractory to at least two proteasome inhibitors, two immunomodulatory agents and an anti-CD38 monoclonal antibody, and who have demonstrated disease progression on the last therapy.[1]
Dosage
It is taken twice per week at a dose of 80 mg each time.[2]
Side effects
In clinical trials, it was associated with a high incidence of severe side effects, including low platelet counts and low blood sodium levels.[8][9][12]
In the clinical study (the BOSTON study) used to support FDA approval in patients with multiple myeloma after at least one prior therapy (once-weekly selinexor in combination with once-weekly bortezomib and dexamethasone),the most common adverse reactions were cytopenias, along with gastrointestinal and constitutional symptoms and were consistent with those previously reported from other selinexor studies. Most adverse reactions were manageable with dose modifications and/or standard supportive care. The most common non-hematologic adverse reactions were fatigue (59%), nausea (50%), decreased appetite (35%), and diarrhea (32%) and were mostly Grade 1 and 2 events. The most common Grade 3 and 4 adverse reactions were thrombocytopenia (43%), lymphopenia (38%), fatigue (28%) and anemia (17%).[13]
The most common adverse reactions (incidence ≥20%) in people with diffuse large B-cell lymphoma (DLBCL), excluding laboratory abnormalities, were fatigue, nausea, diarrhea, appetite decrease, weight decrease, constipation, vomiting, and pyrexia.[11] Grade 3-4 laboratory abnormalities in ≥15% were thrombocytopenia, lymphopenia, neutropenia, anemia, and hyponatremia.[11] Serious adverse reactions occurred in 46% of people, most often from infection.[11] Thrombocytopenia was the leading cause of dose modifications.[11] Gastrointestinal toxicity developed in 80% of people and any grade hyponatremia developed in 61%.[11] Central neurological adverse reactions occurred in 25% of people, including dizziness and mental status changes.[11]
The prescribing information provides warnings and precautions for thrombocytopenia, neutropenia, gastrointestinal toxicity, hyponatremia, serious infection, neurological toxicity, and embryo-fetal toxicity.[11][3]
Mechanism of action
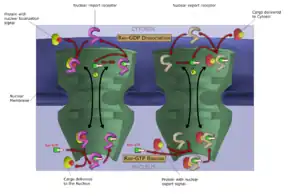
Like other selective inhibitors of nuclear export (SINEs), selinexor works by binding to exportin 1 (also known as XPO1 or CRM1). XPO1 is a karyopherin which performs nuclear transport of several proteins, including tumor suppressors, oncogenes, and proteins involved in governing cell growth, from the cell nucleus to the cytoplasm; it is often overexpressed and its function misregulated in several types of cancer.[14] By inhibiting the XPO1 protein, SINEs lead to a buildup of tumor suppressors in the nucleus of malignant cells and reduce levels of oncogene products which drive cell proliferation. This ultimately leads to cell cycle arrest and death of cancer cells by apoptosis.[14][15][3] In vitro, this effect appeared to spare normal (non-malignant) cells.[14][16]
Inhibiting XPO1 affects many different cells in the body which may explain the incidence of adverse reactions to selinexor.[15] Thrombocytopenia, for example, is a mechanistic and dose-dependent effect, occurring because selinexor causes a buildup of the transcription factor STAT3 in the nucleus of hematopoietic stem cells, preventing their differentiation into mature megakaryocytes (platelet-producing cells) and thus slowing production of new platelets.[15]
Chemistry
Selinexor is a fully synthetic small-molecule compound, developed by means of a structure-based drug design process known as induced-fit docking. It binds to a cysteine residue in the nuclear export signal groove of exportin 1. Although this bond is covalent, it is slowly reversible.[14]
History
Selinexor was developed by Karyopharm Therapeutics, a pharmaceutical company focused on the development of drugs that target nuclear transport. It was approved in the United States in July 2019,[17][18][19] on the basis of a single-arm Phase IIb clinical trial. The FDA decided to grant accelerated approval despite a previous recommendation from an FDA Advisory Committee Panel which had voted 8–5 to delay approving the drug until the results from an ongoing Phase III study were known.[8]
Selinexor in combination with dexamethasone was granted accelerated approval and was granted orphan drug designation.[18] The FDA granted the approval of Xpovio to Karyopharm Therapeutics.[18]
In June 2020, the U.S. Food and Drug Administration (FDA) approved an additional indication for selinexor to treat adults with relapsed or refractory diffuse large B-cell lymphoma (DLBCL), not otherwise specified, including DLBCL arising from follicular lymphoma, after at least two lines of systemic therapy.[11]
Approval was based on SADAL (KCP-330-009; NCT02227251), a multicenter, single-arm, open-label trial in participants with DLBCL after two to five systemic regimens.[11] Participants received selinexor 60 mg orally on days one and three of each week.[11]
In December 2020, the FDA expanded selinexor's approved indication to include its combination with bortezomib and dexamethasone for the treatment of adults with multiple myeloma who have received at least one prior therapy.
Selinexor was granted accelerated approval by the U.S. Food and Drug Administration (FDA) in July 2019, for use in combination with the corticosteroid dexamethasone for the treatment of adults with relapsed refractory multiple myeloma (RRMM) who have received at least four prior therapies and whose disease is resistant to several other forms of treatment, including at least two proteasome inhibitors, at least two immunomodulatory agents, and an anti-CD38 monoclonal antibody.[18] In December 2020, selinexor was approved by the FDA in combination with bortezomib and dexamethasone for the treatment of adults with multiple myeloma who have received at least one prior therapy.[20] It is the first drug with this mechanism of action.[15] [8] The U.S. Food and Drug Administration (FDA) considers it to be a first-in-class medication.[21]
Society and culture
Legal status
On 28 January 2021, the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) adopted a positive opinion, recommending the granting of a conditional marketing authorization for the medicinal product Nexpovio intended for the treatment of relapsed and refractory multiple myeloma.[22] The applicant for this medicinal product is Karyopharm Europe GmbH.[22]
Research
Under the codename KPT-330, selinexor was tested in several preclinical animal models of cancer, including pancreatic cancer, breast cancer, non-small-cell lung cancer, lymphomas, and acute and chronic leukemias.[23] In humans, early clinical trials (phase I) have been conducted in non-Hodgkin lymphoma, blast crisis, and a wide range of advanced or refractory solid tumors, including colon cancer, head and neck cancer, melanoma, ovarian cancer, and prostate cancer.[23] Compassionate use in patients with acute myeloid leukemia has also been reported.[23]
The pivotal clinical trial which served to support approval of selinexor for people with relapsed/refractory multiple myeloma was an open-label study of 122 patients known as the STORM trial.[3] In all of the enrolled patients, patients had been treated with a median of seven prior treatment regimens including conventional chemotherapy, targeted therapy with bortezomib, carfilzomib, lenalidomide, pomalidomide, and a monoclonal antibody (daratumumab or isatuximab);[7] nearly all had also undergone hematopoietic stem cell transplantation but had disease that continued to progress.[3] The overall response rate was 26%, including two stringent complete responses; 39% of patients had a minimal response or better. The median duration of response was 4.4 months, median progression-free survival was 3.7 months, and median overall survival was 8.6 months.[24]
References
- 1 2 3 4 5 6 7 8 "Nexpovio EPAR". European Medicines Agency (EMA). 25 January 2021. Archived from the original on 2 June 2021. Retrieved 27 May 2021. Text was copied from this source which is © European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- 1 2 3 4 5 6 7 8 9 "Selinexor Monograph for Professionals". Drugs.com. Archived from the original on 14 August 2020. Retrieved 12 October 2021.
- 1 2 3 4 5 "Xpovio- selinexor tablet, film coated". DailyMed. 19 August 2019. Archived from the original on 29 August 2021. Retrieved 20 November 2019.
- ↑ "Xpovio Prices, Coupons & Patient Assistance Programs". Drugs.com. Archived from the original on 23 April 2021. Retrieved 12 October 2021.
- ↑ "Selinexor". SPS - Specialist Pharmacy Service. 19 June 2017. Archived from the original on 13 October 2021. Retrieved 12 October 2021.
- ↑ "Archive copy". Archived from the original on 8 March 2021. Retrieved 1 July 2021.
{{cite web}}: CS1 maint: archived copy as title (link) - 1 2 Chim CS, Kumar SK, Orlowski RZ, Cook G, Richardson PG, Gertz MA, et al. (February 2018). "Management of relapsed and refractory multiple myeloma: novel agents, antibodies, immunotherapies and beyond". Leukemia. 32 (2): 252–262. doi:10.1038/leu.2017.329. PMC 5808071. PMID 29257139.
- 1 2 3 4 Feuerstein A (3 July 2019). "FDA approves new multiple myeloma drug despite toxicity concerns". STAT. Archived from the original on 25 September 2020. Retrieved 6 July 2019.
- 1 2 Mulcahy N (3 July 2019). "FDA Approves Selinexor for Refractory Multiple Myeloma". Medscape. Archived from the original on 15 January 2021. Retrieved 6 July 2019.
- ↑ Barrett J (3 July 2019). "New Treatment for Refractory Multiple Myeloma Granted FDA Approval". Pharmacy Times. Archived from the original on 12 August 2020. Retrieved 7 July 2019.
- 1 2 3 4 5 6 7 8 9 10 11 "FDA approves selinexor for relapsed/refractory diffuse large B-cell ly". U.S. Food and Drug Administration. 22 June 2020. Archived from the original on 12 August 2020. Retrieved 24 June 2020.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - ↑ "Drug Trials Snapshots: Xpovio". U.S. Food and Drug Administration (FDA). 16 July 2019. Archived from the original on 20 November 2019. Retrieved 20 November 2019.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - ↑ "Archive copy" (PDF). Archived (PDF) from the original on 28 July 2021. Retrieved 1 July 2021.
{{cite web}}: CS1 maint: archived copy as title (link) - 1 2 3 4 Fung HY, Chook YM (August 2014). "Atomic basis of CRM1-cargo recognition, release and inhibition". Seminars in Cancer Biology. 27: 52–61. doi:10.1016/j.semcancer.2014.03.002. PMC 4108548. PMID 24631835.
- 1 2 3 4 Gandhi UH, Senapedis W, Baloglu E, Unger TJ, Chari A, Vogl D, Cornell RF (May 2018). "Clinical Implications of Targeting XPO1-mediated Nuclear Export in Multiple Myeloma". Clinical Lymphoma, Myeloma & Leukemia. 18 (5): 335–345. doi:10.1016/j.clml.2018.03.003. PMID 29610030.
- ↑ Chen C, Siegel D, Gutierrez M, Jacoby M, Hofmeister CC, Gabrail N, et al. (February 2018). "Safety and efficacy of selinexor in relapsed or refractory multiple myeloma and Waldenstrom macroglobulinemia". Blood. 131 (8): 855–863. doi:10.1182/blood-2017-08-797886. PMID 29203585.
- ↑ "Drug Approval Package: Xpovio". U.S. Food and Drug Administration (FDA). 26 July 2019. Archived from the original on 9 April 2021. Retrieved 24 June 2020.
- 1 2 3 4 "FDA approves new treatment for refractory multiple myeloma". U.S. Food and Drug Administration (FDA) (Press release). 3 July 2019. Archived from the original on 20 November 2019. Retrieved 20 November 2019.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - ↑ "Xpovio (selinexor) FDA Approval History". Drugs.com. 3 July 2019. Archived from the original on 9 July 2019. Retrieved 20 November 2019.
- ↑ "Archive copy". Archived from the original on 8 March 2021. Retrieved 1 July 2021.
{{cite web}}: CS1 maint: archived copy as title (link) - ↑ "New Drug Therapy Approvals 2019". U.S. Food and Drug Administration. 31 December 2019. Archived from the original on 16 September 2020. Retrieved 15 September 2020.
- 1 2 "Nexpovio: Pending EC decision". European Medicines Agency (EMA). 29 January 2021. Archived from the original on 1 February 2021. Retrieved 1 February 2021. Text was copied from this source which is © European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- 1 2 3 Parikh K, Cang S, Sekhri A, Liu D (October 2014). "Selective inhibitors of nuclear export (SINE)--a novel class of anti-cancer agents". Journal of Hematology & Oncology. 7: 78. doi:10.1186/s13045-014-0078-0. PMC 4200201. PMID 25316614.
- ↑ Chari A, Vogl DT, Gavriatopoulou M, Nooka AK, Yee AJ, Huff CA, et al. (August 2019). "Oral Selinexor-Dexamethasone for Triple-Class Refractory Multiple Myeloma". The New England Journal of Medicine. 381 (8): 727–738. doi:10.1056/NEJMoa1903455. PMID 31433920.
External links
| External sites: |
|
|---|---|
| Identifiers: |