Sodium selenate
 | |
| Names | |
|---|---|
| IUPAC name
Sodium selenate | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.033.169 |
| EC Number |
|
PubChem CID |
|
| RTECS number |
|
| UNII |
|
| UN number | 2630 |
CompTox Dashboard (EPA) |
|
InChI
| |
SMILES
| |
| Properties | |
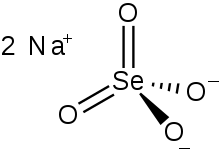
Chemical formula |
Na2O4Se |
| Molar mass | 188.947 g·mol−1 |
| Appearance | White or grey powder |
| Density | 3.098 g/cm3 |
Solubility in water |
soluble |
| Pharmacology | |
| A12CE01 (WHO) | |
| Hazards | |
| GHS labelling: | |
Pictograms |
   |
Signal word |
Danger |
Hazard statements |
H301, H331, H373, H410 |
| NFPA 704 (fire diamond) | |
| Flash point | Non-flammable |
| Related compounds | |
Other anions |
Sodium sulfate |
Other cations |
Potassium selenate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Sodium selenate is the inorganic compound with the formula Na
2SeO
4, not to be confused with sodium selenite. It exists as the anhydrous salt, the heptahydrate, and the decahydrate.[1] These are white, water-soluble solids. The decahydrate is a common ingredient in multivitamins and livestock feed as a source of selenium. The anhydrous salt is used in the production of some glass. Although the selenates are much more toxic, many physical properties of sodium selenate and sodium sulfate are similar.[2]
Production
Sodium selenate is produced by oxidation of selenium, first with nitric acid, producing selenous acid. The selenous acid is neutralized to form sodium selenite. The sodium selenite is oxidized in a basic medium hydrogen peroxide to form a selenate, which is then spray-dried.[3]
- Se + 2HNO3 → H2SeO3 + NO + NO2
- H2SeO3 + Na2CO3 → Na2SeO3 + H2O + CO2
- Na2SeO3 + H2O2 → Na2SeO4 + H2O.
It was prepared shortly after the discovery of selenium by Jöns Jacob Berzelius in 1817.
Industrial uses
Glass manufacturing
One of the earliest applications of sodium selenate was in the glass industry. Selenium produces a red hue in glass. The molten glass is treated with sodium selenate and then arsenic trioxide to reduce the compound and provide elemental selenium. Sodium selenate is also used as a decolorizing agent in glass production. The red hue it gives glass is complementary to the green hue given by ferrous oxides in the manufacturing process. When used together, the two compound produce a colorless glass.[4]
Pesticide
Sodium selenate is a common ingredient in some insecticides used against mites, aphids, and mealybugs. For most insects, a dose of 10 mg/kg is enough to be fatal.[5][6] It is also used in some fungicides.
Bio-fortification of crops
Sodium selenate is effectively used for bio-fortification of crops hence fortifying food/feed to mitigate selenium deficiency in humans and livestock. It can be applied as foliar spray or via rooting medium e.g. added in fertilizers.
Dietary supplement
Chosen for its selenium content and high solubility, sodium selenate is a common ingredient in over-the-counter vitamin supplements. Selenium is a trace essential element. Sodium selenate and selenite are also common in premix animal feed. Neither compound has demonstrated a difference in the amount of selenium absorbed. The U.S. FDA regulates that animal feed contain no more than 5 ppm selenium content.[7] Controversy arose in 2009 when a group of 21 polo horses died from selenium poisoning from an incorrectly mixed dietary supplement.[8]
Toxicology
The U.S. FDA and European Union currently classify sodium selenate as toxic, primarily if ingested or inhaled. Testing on rats showed a dose of 1.6 mg/kg to be lethal. A low lethal dose as this places the chemical as being 2 to 3 times more toxic than sodium cyanide. As such, it is extremely toxic and must be handled with care. For a 70 kg (154 pound) person, this dosage corresponds to 112 mg, or, in terms of 200 µg pills, 560 pills. Chronic exposure to sodium selenate can cause severe lung, kidney, and liver damage.[9]
Overexposure to selenium in the diet leads to a condition known as selenosis. Selenosis occurs at blood levels greater than 100 µg/dL. Symptoms include gastrointestinal upsets, hair loss, white blotchy nails, garlic breath odor, fatigue, irritability, and mild nerve damage.[10]
References
- ↑ Kamburov, S.; Schmidt, H.; Voigt, W.; Balarew, C. "Similarities and peculiarities between the crystal structures of the hydrates of sodium sulfate and selenate" Acta Crystallographica B (Struct Sci Cryst Eng Mater.) 2014, volume 70, pp. 714-22. doi:10.1107/S2052520614007653
- ↑ Bernd E. Langner "Selenium and Selenium Compounds" in Ullmann's Encyclopedia of Industrial Chemistry, 2005, Wiley-VCH, Weinheim. doi:10.1002/14356007.a23_525.
- ↑ Bjornberg, A.; Martensson, U.S.; Paulsson K.M. (Boliden Aktiebolag). Method for producing selenium salts. US Patent 4,605,544, August 12, 1986.
- ↑ Whitaker, M.C.; Journal of Industrial and Engineering Chemistry. 1912, 7, 4. pg. 539-540
- ↑ Krieger, K. Handbook of Pesticide Toxicology: Volume 1; Academic Press: San Diego, CA, 2001
- ↑ Hanson, B.; Lindblom, S.D.; Loeffler, M. L.; Pilon-Smits, E.; New Phytologist. 2004, 3, 162. Pg. 655-662.
- ↑ Podoll, K.L.; Bernard, J.B.; Ullrey, D.E.; DeBar, S.R.; Ku, P.K.; Magee, W.T.; Journ. of Animal Sci. 1992, 70, 6. P. 1965-1970.
- ↑ Shipley, Amy "Polo Horse’s Death Came From Incorrectly Mixed Supplement" The Washington Post, Washington, April 29, 2009, p 1.
- ↑ Ganther, H.E.; Baumann, C.A.; The Journ. of Nutrition. 1962, 77. P. 408-414
- ↑ Dietary Supplement Fact Sheet: Selenium. http://ods.od.nih.gov/factsheets/selenium (accessed 10/19/2011).
